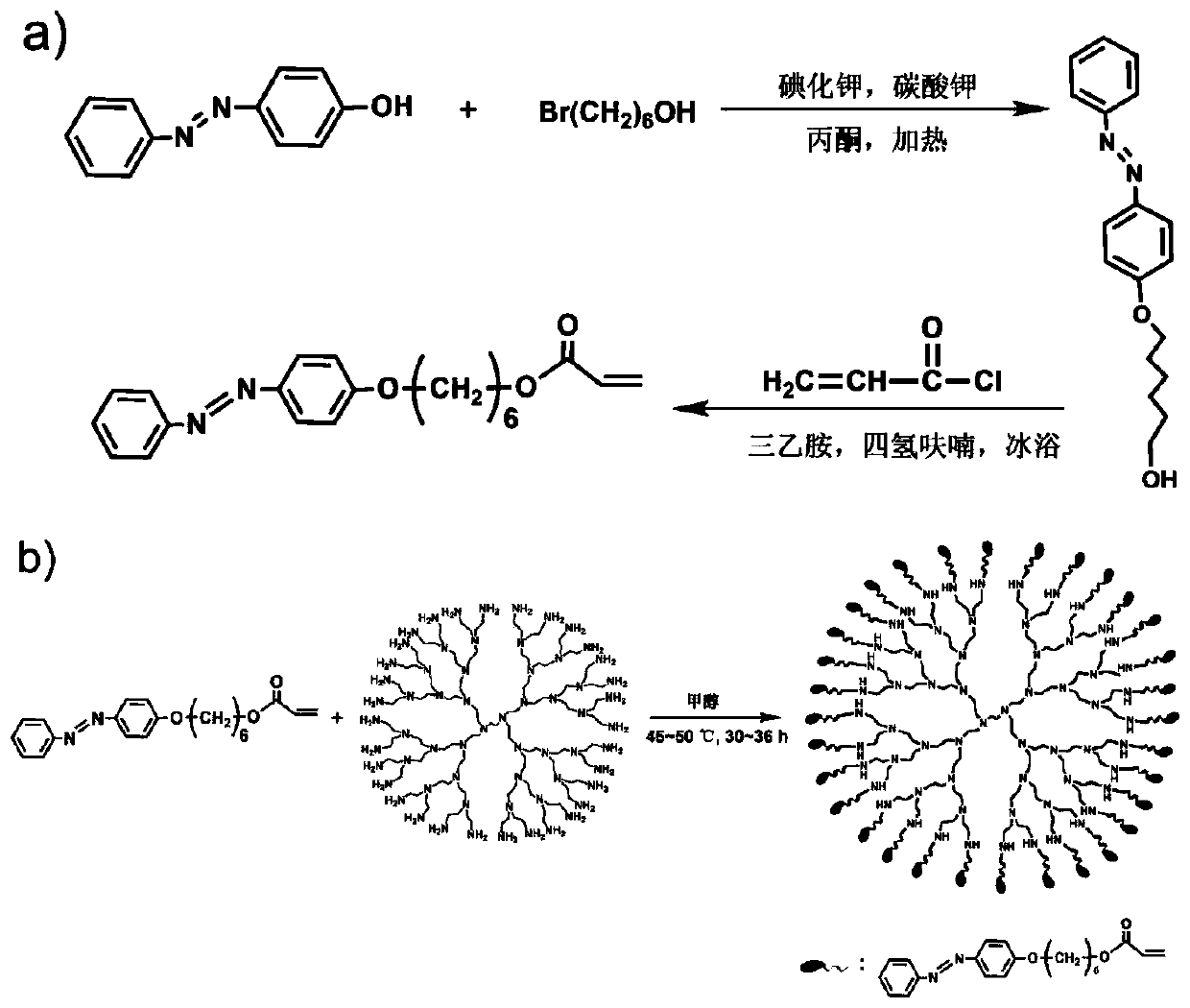
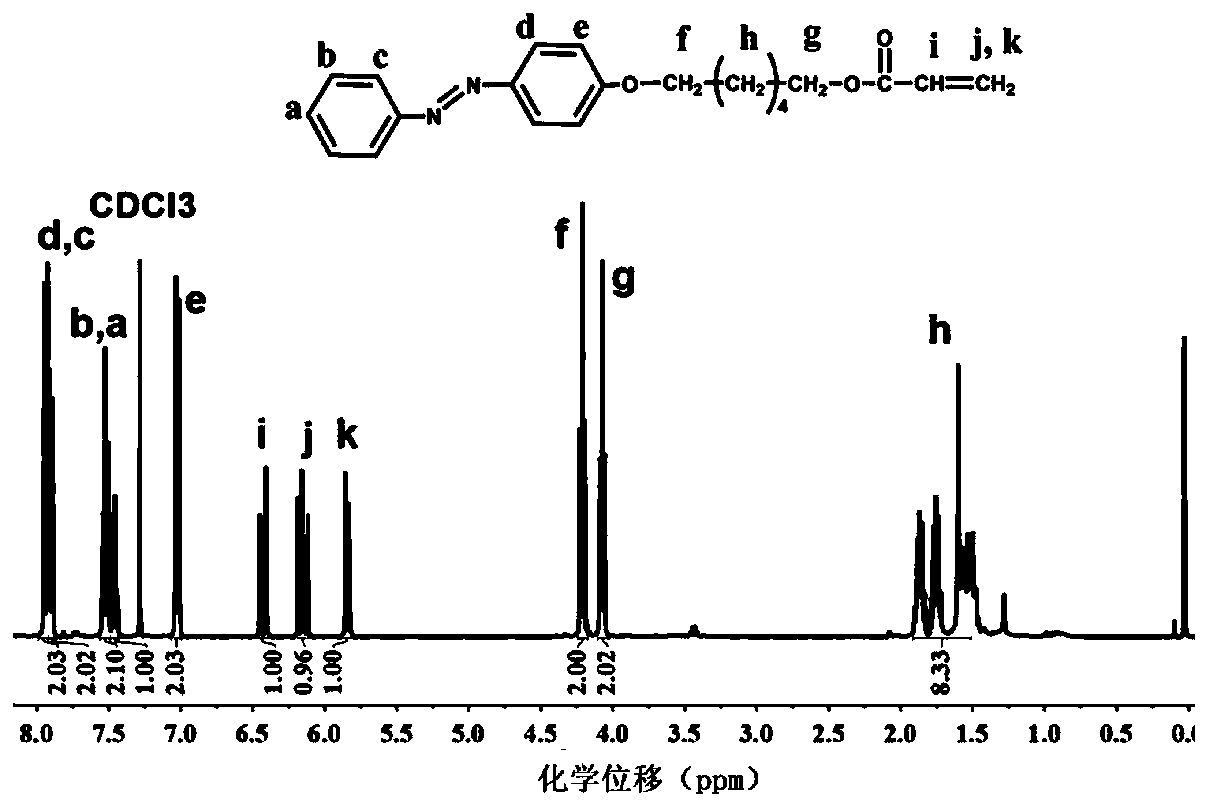
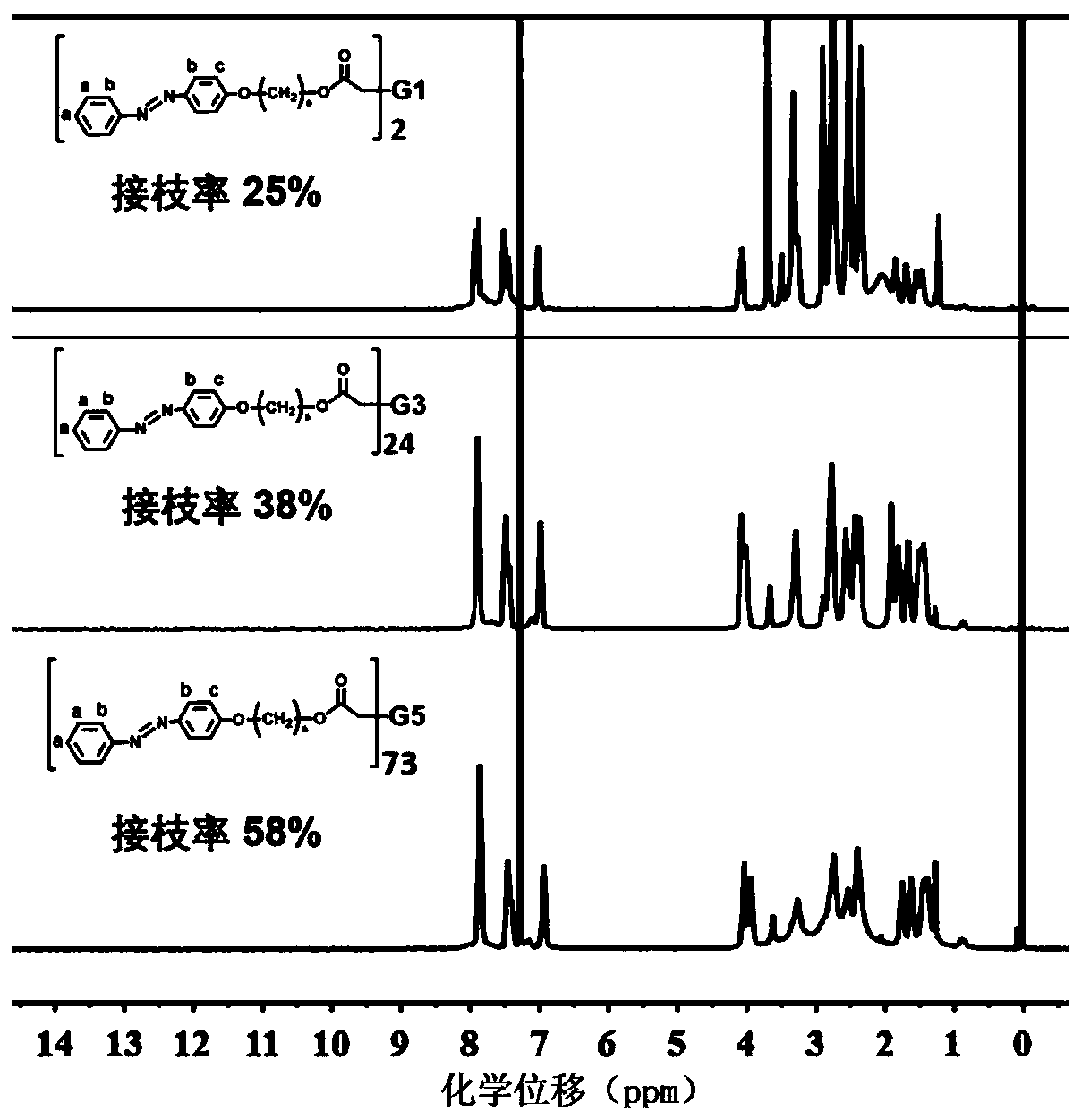
Method for preparing light-controlled solid-liquid transformed azo polymer
An azo polymer, solid-liquid transformation technology, applied in chemical instruments and methods, heat exchange materials, etc., can solve problems such as insufficient fluidity of solid polymer chains, achieve high molecular weight, facilitate room temperature processing, and film-forming properties Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) 4-hydroxyazobenzene (10g, 50mmol), potassium iodide (4.15g, 25mmol), potassium carbonate (69g, 500mmol) were added to 150ml acetone and stirred, then 6-bromo-1-hexanol was added dropwise ( 13g, 70mmol) in acetone (50ml). The mixture was heated to reflux in an oil bath at 65°C for 24 hours to obtain a mixed solution A, and the mixed solution A was poured into 1000ml of cold water to precipitate the product and remove inorganic salts, and the filtered solid was vacuum-dried at 40°C for 30 hours to obtain intermediate product. The above intermediate product (6.2g, 20mmol) and triethylamine (9.8ml, 70mmol) were dissolved in 100ml tetrahydrofuran and stirred in an ice bath for 30min, then 20ml acryloyl chloride (4.8ml, 60mmol) was slowly added dropwise. Continue to stir the reaction mixture in an ice bath for 4 hours to obtain a mixed solution B, pour the mixed solution B into 1000ml of cold water to precipitate the product while removing excess acryloyl chloride and tri...
Embodiment 2
[0030] 1) Add 4-hydroxyazobenzene (3.96g, 20mmol), potassium iodide (2g, 12mmol), potassium carbonate (35g, 250mmol) into 100ml acetone and stir, then add 6-bromo-1-hexanol dropwise ( 6.25g, 35mmol) in acetone (30ml). The mixture was heated under reflux in an oil bath at 68°C for 28 hours to obtain a mixed solution A, and the mixed solution A was poured into 1200ml of cold water to precipitate the product and remove inorganic salts, and the filtered solid was vacuum-dried at 45°C for 36 hours to obtain intermediate product. The above intermediate product (3.1g, 10mmol) and triethylamine (5ml, 36mmol) were dissolved in 80ml tetrahydrofuran and stirred in an ice bath for 20min, then 15ml acryloyl chloride (2ml, 24mmol) was slowly added dropwise. Continue to stir the reaction mixture in an ice bath for 5 hours to obtain a mixed solution B. Pour the mixed solution B into 1200ml of cold water to precipitate the product while removing excess acryloyl chloride and triethylamine salt...
Embodiment 3
[0033] 1) Add 4-hydroxyazobenzene (5g, 25mmol), potassium iodide (2.2g, 13mmol), potassium carbonate (25g, 180mmol) into 150ml acetone and stir, then add 6-bromo-1-hexanol dropwise ( 5g, 27mmol) in acetone (20ml). The mixture was heated under reflux in an oil bath at 70°C for 36 hours to obtain a mixed solution A, and the mixed solution A was poured into 1800ml of cold water to precipitate the product and remove inorganic salts, and the filtered solid was vacuum-dried at 48°C for 40 hours to obtain intermediate product. The above intermediate product (2.5g, 8mmol) and triethylamine (2.4ml, 30mmol) were dissolved in 50ml of tetrahydrofuran and stirred in an ice bath for 10min, then 15ml of acryloyl chloride (2ml, 24mmol) was slowly added dropwise. Continue to stir the reaction mixture in an ice bath for 5 hours to obtain a mixed solution B. Pour the mixed solution B into 1000ml of cold water to precipitate the product while removing excess acryloyl chloride and triethylamine s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com