Method for synthesizing 9beta,10alpha-dehydroprogesterone ketal through photochemical isomerization in micro-channel reactor
A technology of microchannel reactor and photochemical isomerization, which is applied in chemical instruments and methods, chemical/physical/physical chemical processes, chemical/physical/physical chemical reactors, etc. Low mass and heat transfer efficiency, unstable light and other problems, to achieve the effect of inhibiting the formation of by-products of photoisomerization reaction, convenient and feasible industrial production, and long service life of light source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
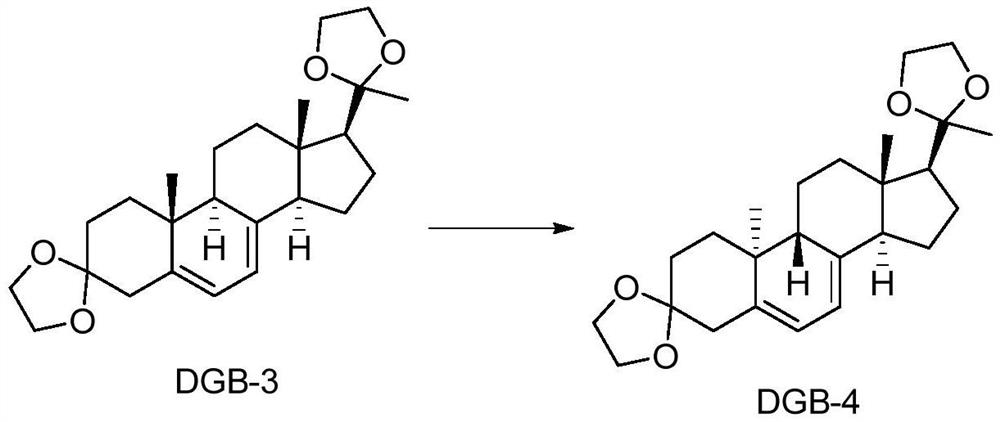
[0045] (1) 9β,10α-Dehydroprogesterone ketal photochemical deactivation reaction
[0046] Add 1L of ethyl acetate to a 3L round bottom flask, then add 30g of raw material 9α,10β-dehydroprogesterone ketal and stir to dissolve, then add 3.8mg of triethylamine and 12.5mg of 2,6-di-tert-butyl p-methyl base phenol, stirred and mixed evenly, and prepared into a photochemical reaction solution.
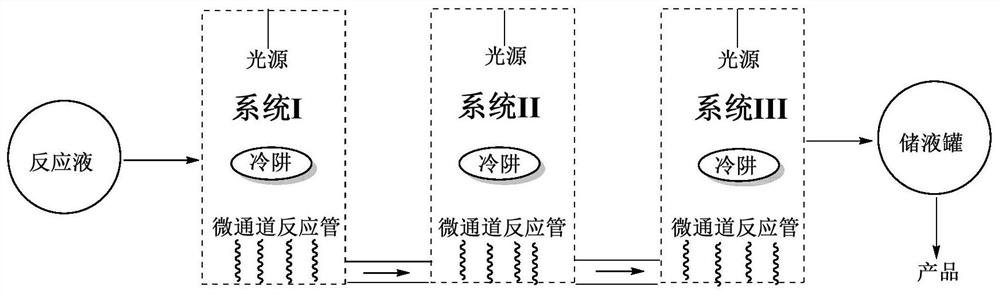
[0047] Into the obtained photochemical reaction solution, pass into nitrogen to protect, open the 500W high-pressure mercury lamp placed in the cold trap inner cavity in the microchannel photoreactor system I after 30 minutes, and open the cooling water valve of the system I, in the described While the cold trap is cooling down, the photochemical reaction solution is pumped into the micro-flow tube wound on the outer wall of the cold trap in system I by a peristaltic pump at a speed of 5mL / min to carry out the light reaction until the photochemical reaction solution passes through the After ...
Embodiment 2
[0054] (1) 9β,10α-Dehydroprogesterone ketal photochemical deactivation reaction
[0055] Add 1L of acetone to a 3L round bottom flask, then add 25g of raw material 9α, 10β-dehydroprogesterone ketal and stir to dissolve, then add 6.7mg of tert-butyl hydroquinone and 3.5mg of pyridine, stir and mix evenly, and prepare Photochemical reaction solution.
[0056] Into the obtained photochemical reaction solution, pass into nitrogen to protect, open the 500W high-pressure mercury lamp placed in the cold trap inner cavity in the microchannel photoreactor system I after 30 minutes, and open the cooling water valve of the system I, in the described While the cold trap is cooling down, the photochemical reaction solution is pumped into the micro-flow tube wound on the outer wall of the cold trap in the system I by a peristaltic pump at a speed of 6 mL / min to carry out the light reaction until the photochemical reaction solution passes through the After the system I is illuminated, it is...
Embodiment 3
[0063] (1) 9β,10α-Dehydroprogesterone ketal photochemical deactivation reaction
[0064] Add 300ml tetrahydrofuran to a 1L round bottom flask, then add 10g raw material 9α,10β-dehydroprogesterone ketal and stir to dissolve, then add 8.3mg 2,6-di-tert-butyl-p-cresol and 4.5mg tert-butyl Potassium alkoxide, stirred and mixed evenly, prepared into a photochemical reaction liquid.
[0065] Into the obtained photochemical reaction liquid, feed nitrogen to protect, open the 1000W high-pressure mercury lamp placed in the cold trap inner cavity in the microchannel photoreactor system 1 after 30 minutes, and open the cooling water valve of the system 1, in the described While the cold trap is cooling down, the photochemical reaction solution is pumped into the micro-flow tube wound on the outer wall of the cold trap in system I by a peristaltic pump at a speed of 5mL / min to carry out the light reaction until the photochemical reaction solution passes through the After the system I is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com