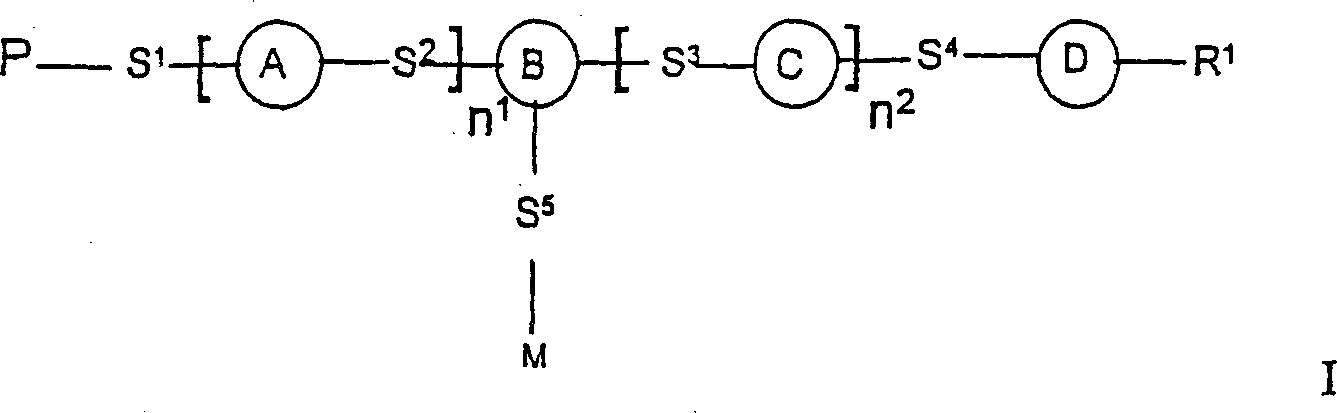
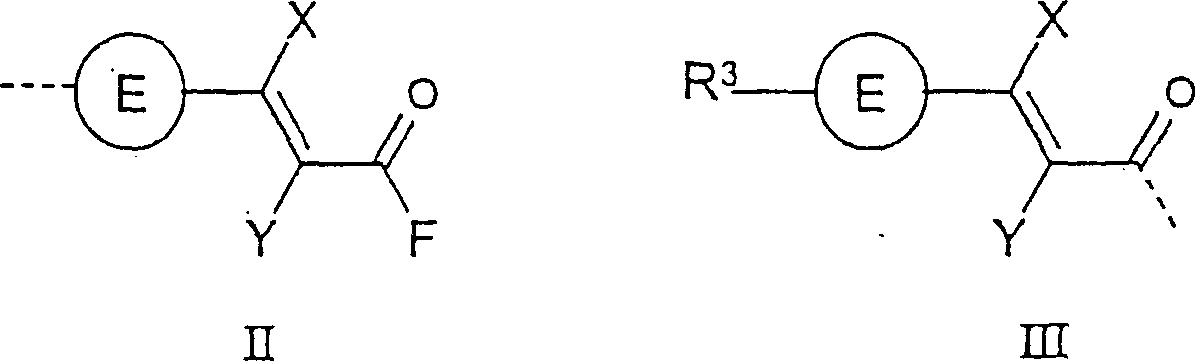
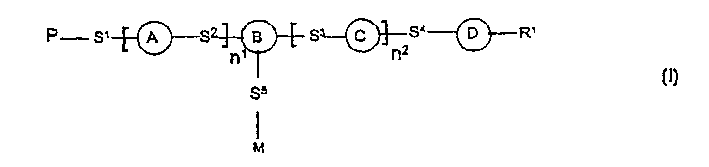Photoactive polymers
A photoactive polymer, photodimerization technology, applied in optics, optomechanical equipment, nonlinear optics, etc., can solve the problem of high surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Poly[1-[11-[5-[4-[(E)-2-methoxycarbonylvinyl]benzoyloxy]-2-(4-propylbenzoyloxy)benzoyl Oxy]undecyloxycarbonyl]-1-methylethylene]
[0213]
[0214] In a sealed tube, 0.89 g (1.22 mmol) of (E)-5-[4-(2-methoxycarbonylvinyl)benzoyloxy]- 11-(2-methacryloyloxy)undecyl 2-(4-propylbenzoyloxy)benzoate and 2.0 mg (0.012 mmol) α,α′-azobisisobutyronitrile (AIBN) mixture was degassed. The tube was then sealed under argon and stirred at 60°C for 17 hours. The polymer was diluted with 2.5 ml THF, precipitated in 500 ml diethyl ether and collected. Dissolution in THF (7.0 ml) and reprecipitation of the polymer in 750 ml of methanol afforded 0.71 g (80%) of poly[1-[11-[5-[4-[(E)-2 -Methoxycarbonylvinyl]benzoyloxy]-2-(4-propylbenzoyloxy)benzoyloxy]undecyloxycarbonyl]-1-methylethylene]; Mn =7.4×10 4 , pdi=6.95, Tg=49.5°C, cl.p.(N / I) 127.3°C.
[0215] (E)-5-[4-(2-Methoxycarbonylvinyl)benzoyloxy]-2-(4-propylbenzoyloxy)benzoic acid was prepared as a starting material as follows 11...
Embodiment 2
[0239] Poly[1-[11-[2-[4-pentylbenzoyloxy]-5-[6-[2-methoxy-4-(methoxycarbonylvinyl)phenoxy]oxy Hexyl]benzoyloxy]deca-alkoxycarbonyl]-1-methylethylene]
[0240]
[0241] With 1.0g (1.17mmol) (E)-2-[4-phenylbenzoyloxy]-5-[6-[2-methoxy-4-(methoxycarbonylvinyl)phenoxy ] Oxyhexyl] 11-(2-methacryloyloxy) undecyl benzoate, prepared in a similar manner to Example 1, to obtain 0.84 g (84%) of hard solid poly[1-[ 11-[2-[4-Pentylbenzoyloxy]-5-[6-[2-methoxy-4-(methoxycarbonylvinyl)phenoxy]oxyhexyl]benzoyl Oxygen]undecyloxycarbonyl]-1-methylethylene]; Mn=7.3×10 4 , pdi=4.40, Tg=34.0°C, cl.p(N / I) 54.2°C.
[0242] (E)-2-[4-phenylbenzoyloxy]-2-[5-[2-methoxy-4-(methoxycarbonylvinyl)benzene used as starting material was prepared as follows Oxy]oxyhexyl]benzoic acid 11-(2-methacryloyloxy)undecyl ester:
[0243] (E)-4-Hydroxy-3-methoxycinnamic acid methyl ester
[0244]
[0245] 25 g (0.13 mmol) of (E)-4-hydroxy-3-methoxycinnamic acid were dissolved in 180 ml of methanol, and 5 ml of c...
Embodiment 3
[0256] Polyimide
[0257] To 0.434g (0.547mmol) 3,5-diaminobenzoic acid 11-[2-[4-pentylbenzoyloxy]-5-[4-(2-methoxycarbonylvinyl)benzoyl To a solution of oxy]benzoyloxy]undecyl ester in 3 ml of tetrahydrofuran was added 96.6 mg (0.493 mmol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride. It was then stirred at 0°C for 2 hours. 10.7 mg (0.055 mmol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride were added. The mixture was then allowed to react at room temperature for 69 hours. The polymer mixture was diluted with 3.0 ml THF, precipitated in 150 ml diethyl ether and collected. The polymer was added from THF (10ml) into 500ml of water for reprecipitation, and after vacuum drying at room temperature, 0.51g of light brown powdery polyamic acid A was obtained; [η]=0.51dL / g.
[0258] 3,5-diaminobenzoic acid 11-[2-[4-pentylbenzoyloxy]-5-[4-(2-methoxycarbonylvinyl)benzene as starting material was prepared as follows Formyloxy]benzoyloxy]undecyl ester:
[0259] 11-Bromoundecy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com