Quick-response reversible-photoisomerization perfluorinated-ether-chain azobenzene and preparation method therefor
A perfluoroether chain azobenzene, photoisomerization technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problem of slow light response speed and light response degree of photochromic azobenzene compounds Weak and other problems, to achieve the effect of rapid, complete and reversible response, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
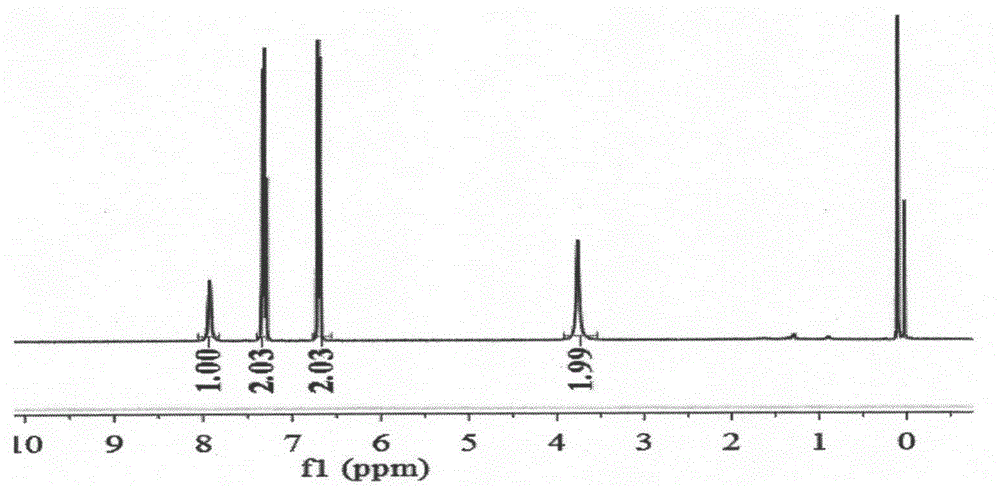
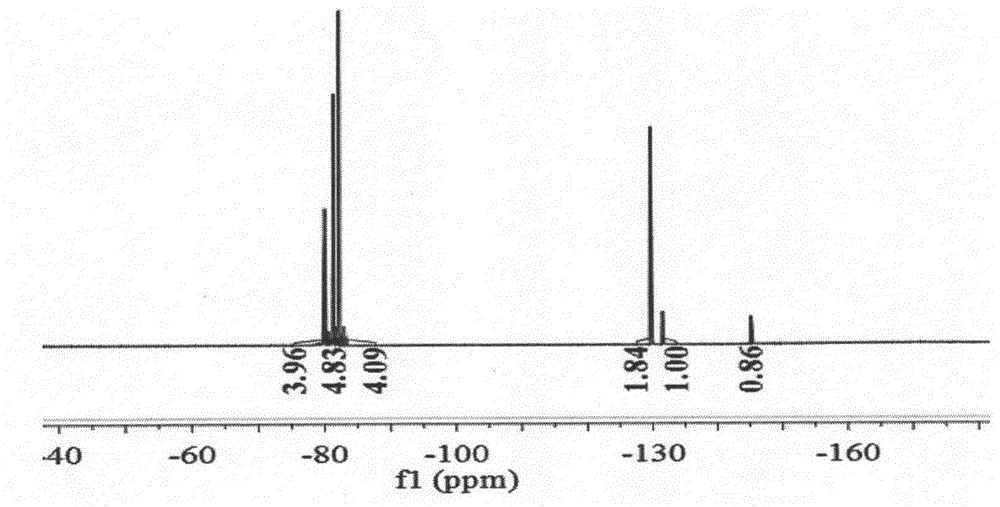
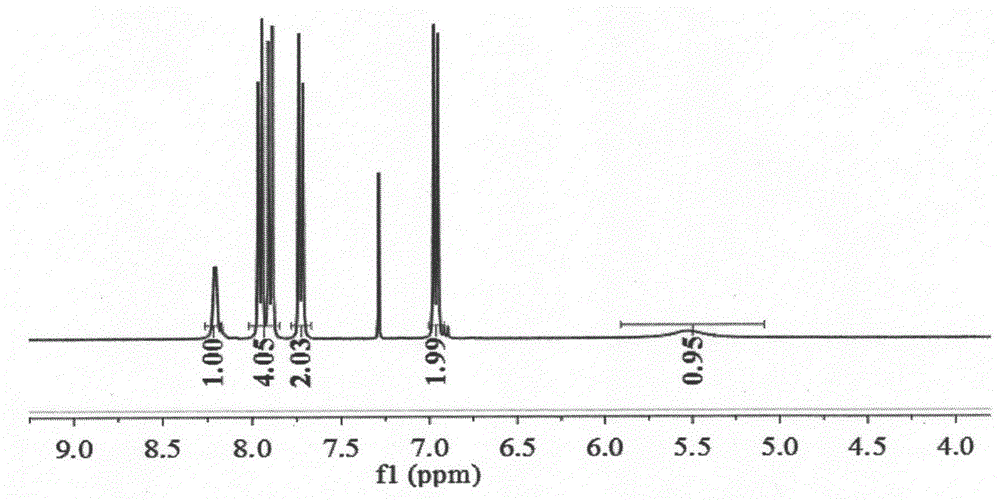
[0032] (1) The three-necked flask equipped with a reflux device was subjected to anhydrous treatment, and under ice bath conditions (0-5°C), a mixture of p-phenylenediamine (2.16g, 20mmol) and triethylamine (4mL, 30mmol) was added 60 mL of tetrahydrofuran solution was stirred with magnetic force for 30 min, then perfluoroether fluoride was added dropwise, and added dropwise for 2 hours until the reaction was complete. After the reaction, the reaction was quenched with water, and the organic phase was spin-dried under reduced pressure, and purified by column chromatography to obtain (4-perfluoroetheramide) aniline (R f , n=1, compound a) 3.26g (white solid, yield 55.6%). Its NMR spectrum is as Figure 1a , 1b shown.
[0033] (2) Add 30mL of 2M hydrochloric acid solution dropwise to compound a (1.172g, 2mmol) under ice bath conditions (0-5°C), dissolve under magnetic stirring conditions, and slowly add 30% of Sodium nitrate solution 0.51g, continue to react for 0.5h. Slowly ...
Embodiment 2
[0036] (1) The three-necked flask equipped with a reflux device was subjected to anhydrous treatment, and at room temperature, 30 mL of a dichloromethane solution of p-phenylenediamine (1.08 g, 10 mmol) and triethylamine (2 mL, 15 mmol) was added, and magnetically After stirring for 30 minutes, perfluoroether-acyl fluoride was added dropwise, and added dropwise for 2 hours until the reaction was complete. After the reaction, the reaction was quenched with water, the organic phase was spin-dried under reduced pressure, and purified by column chromatography to obtain 1.54 g of compound a (white solid, yield 52.6%).
[0037] (2) Add 30mL of 2M hydrochloric acid solution dropwise to compound a (5.86g, 10mmol) under ice bath conditions (0-5°C), dissolve under magnetic stirring conditions, and slowly add 30% of Sodium nitrate solution 2.8g, continue to react for 0.5h. Slowly add a solution of phenol (1.41g, 15mmol) in sodium hydroxide (0.8g, 20mmol) and sodium carbonate (1.48g, 14m...
Embodiment 3
[0039] Example 3: Compound c transforms from trans to cis under UV light irradiation
[0040] Compound c was dissolved in tetrahydrofuran to prepare 5 × 10 -5 mol / L solution, under the irradiation of 365nm ultraviolet light, record the ultraviolet spectrogram every 30s, and draw the absorbance-wavelength relationship graph at different time of ultraviolet light irradiation, such as Figure 4 shown. Figure 4 It shows that compound c rapidly undergoes trans-cis isomerization under the irradiation of 365nm ultraviolet light, and after continuous irradiation for about 120s, the spectrogram no longer changes significantly, and reaches a photostable state, completely converting from trans to cis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com