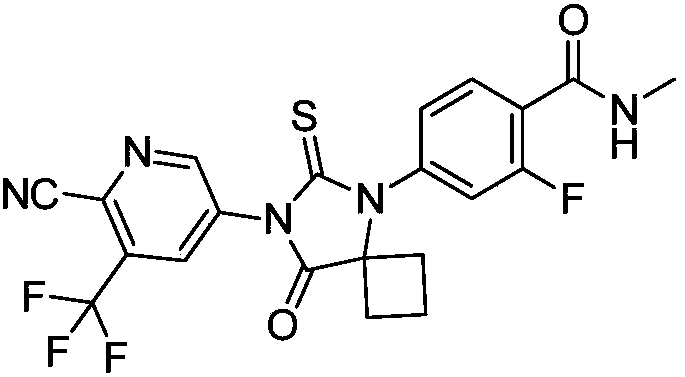
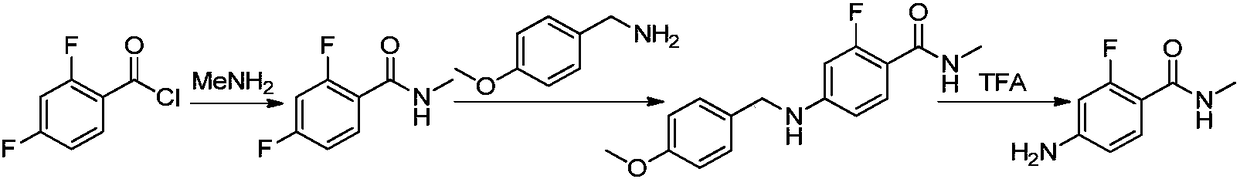
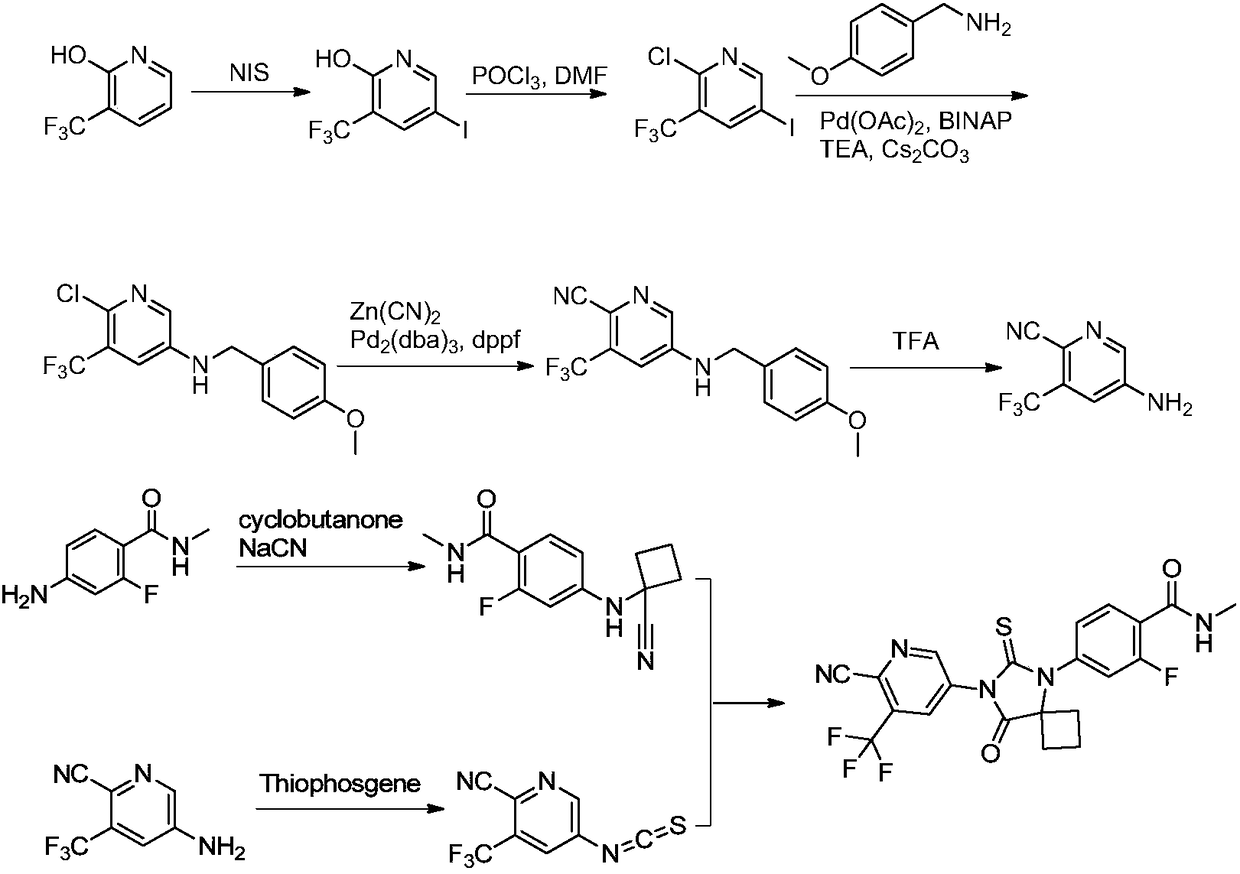
Method for synthesizing apalutamide and intermediate thereof
A technology of apalutamide and a synthetic method, applied in the field of medicine and chemical industry, can solve the problems of the cost of amplifying the production route, long linear steps, complicated process operation, etc., and achieves the advantages of reducing the process cost, reducing the generation of by-products, and simplifying the process operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Add N-methyl-2-fluoro-4-bromo-benzamide 1a (23.21g, 100mmol), cyclobutanine hydrochloride 2 (15.16 100mmol) and N,N-dimethylacetamide into the three-necked flask (116mL), copper iodide (1.90g, 10mmol) and cesium carbonate (71.68g, 220mmol) were added under nitrogen protection, stirred evenly and heated to 90-95°C overnight. After the reaction was completed, water (232 mL) and isopropyl acetate (232 mL) were added, stirred and separated, the aqueous phase was collected and adjusted to pH 3-4 with 4N hydrochloric acid, a large amount of solid was precipitated, and compound 3 (17.88 g, 79%) was obtained by filtration and drying. MS(ESI)m / z=227.2[M+H] + .
[0041] The cuprous iodide here can be replaced by cuprous chloride or cuprous bromide; the alkaline substance cesium carbonate can be replaced by potassium carbonate and sodium carbonate; the solvent N,N-dimethylacetamide can be replaced by N,N-dimethylformamide Amide, N-methylpyrrolidone instead.
[0042]...
Embodiment 2
[0044]
[0045] Add compound 3 (26.63g, 100mmol) and methanol (133mL) into a three-neck flask, stir well, then slowly add thionyl chloride (17.85g, 150mmol), then heat to 40-45°C for 6-8 hours. At the end of the reaction, part of the methanol was spun off, water (266 mL) was slowly added to make a slurry, and filtered. The crude product was then slurried with a mixed solvent of methanol and petroleum ether, filtered and dried to obtain compound 4a (25.79 g, 92%). MS(ESI)m / z=281.2[M+H] +1 H NMR(400MHz,DMSO-d6)δ7.67-7.45(m,2H),6.80(s,1H),6.22-6.41(m,1H),5.98-6.17(m,1H),3.62(s,3H ), 2.82-2.67 (m, 5H), 2.62-2.50 (m, 2H), 2.32-2.26 (m, 1H), 1.82-1.67 (m, 1H) ppm.
Embodiment 3
[0047]
[0048]Add 7 (28.03g, 100mmol) and 75% methanol (280mL) into the three-necked flask, stir well and add potassium thiocyanate (23.00g, 120mmol) and diisopropylethylamine (25.85g, 200mmol). Reflux reaction for 6-8 hours. After the reaction was completed, part of the water was spun off, water (248 mL) was added to make a slurry, recrystallized with a mixed solvent of ethyl acetate and petroleum ether, filtered, and dried to obtain compound 5 (27.56 g, 85%). MS(ESI)m / z=308.0[M+H] +1 HNMR(400MHz,DMSO-d6)δ9.32(br,1H),δ7.70-7.55(m,2H),6.82-6.70(m,1H),6.12(br,1H),2.84-2.65(m, 5H), 2.63-2.50 (m, 2H), 2.35-2.25 (m, 1H), 1.85-1.65 (m, 1H) ppm.
[0049] Here potassium thiocyanate can be replaced by sodium thiocyanate; alkaline substance diisopropylethylamine can be replaced by triethylamine, pyridine, DMAP, DBU or DABCO; solvent methanol can be replaced by N,N-dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, ethanol, isopropanol, acetonitrile, tetrahydrofuran,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com