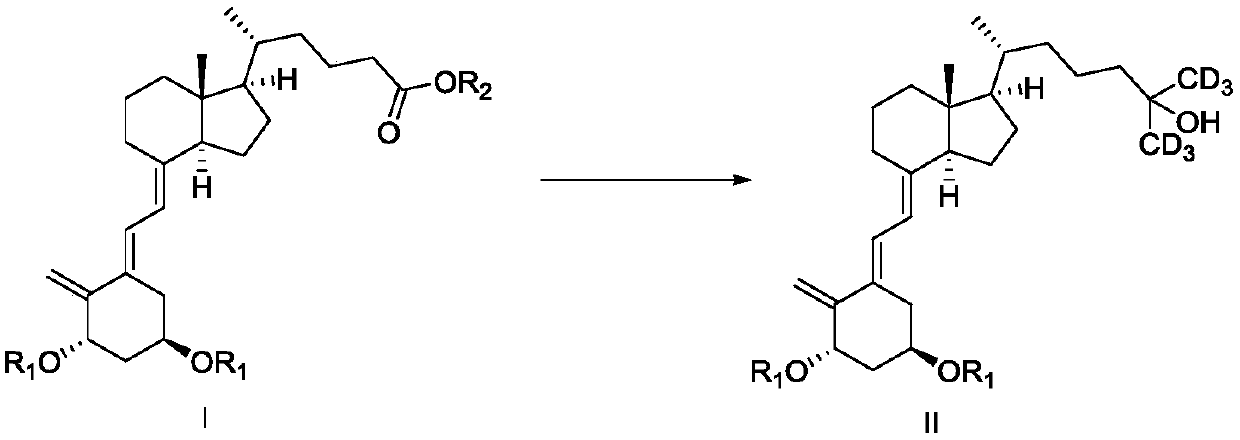
Preparation method and intermediate of deuterated calcitriol
A technology of calcitriol and compounds, which is applied in the field of preparation of deuterated calcitriol, can solve problems such as harsh reaction conditions, use of special reagents, complex separation and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
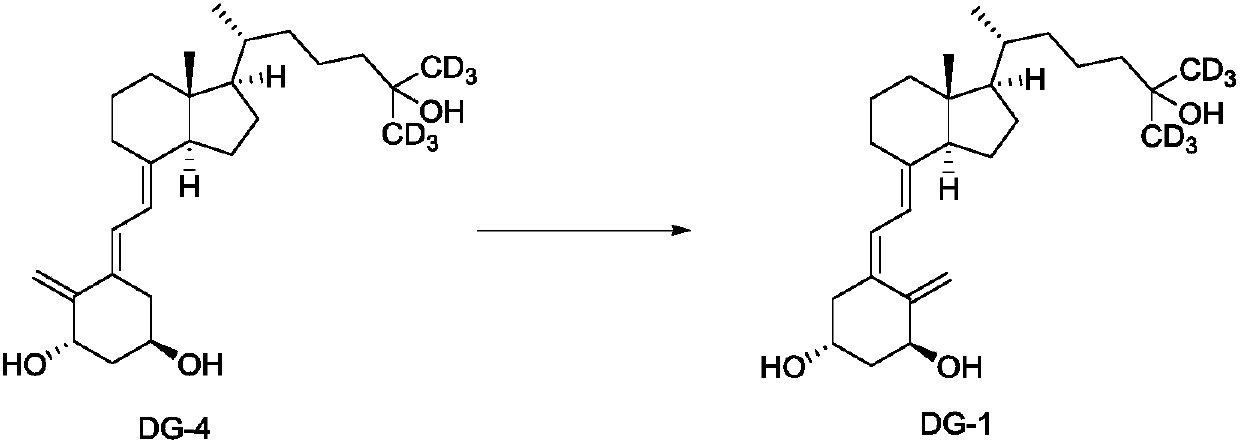
[0095] (1α,3β,5E,7E)-26,27-Hexadeuterio-1,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-9,10-open Preparation of Cyclocholesta-5,7,10(19)-trien-25-ol (DG-3)
[0096] Under an argon atmosphere, DG-2 (3.2g, 4.85mmol) was dissolved in anhydrous tetrahydrofuran (16ml), and deuteromethylmagnesium iodide (CD 3 MgI) in 1.0mol / L ether solution (19.4ml, 19.4mmol). After dropping, the reaction mixture was raised to room temperature and stirred for 3 h. The resulting reaction mixture was cooled to 0 °C and carefully quenched with saturated aqueous ammonium chloride. Ethyl acetate was added for extraction. The organic extract was washed with saturated aqueous sodium chloride and dried over anhydrous sodium sulfate. Concentrate under reduced pressure and dry in vacuo to obtain DG-3 (quantitative yield). The obtained DG-3 does not contain DG-2 and can be directly put into the next reaction without purification. 1 H NMR (400MHz, CDCl 3 )δ: 6.46(d, J=11.5Hz, 1H), 5.83(d, J=11.5Hz, 1H), ...
Embodiment 2
[0098] (1α,3β,5E,7E)-26,27-Hexadeuterio-1,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-9,10-open Preparation of Cyclocholesta-5,7,10(19)-trien-25-ol (DG-3)
[0099] Mix magnesium scraps (1.33g, 54.7mmol) with anhydrous ether (6ml), add deuteromethyl iodide (6.33g, 43.66mmol) in anhydrous ether (26ml) dropwise at 0-5°C in an argon atmosphere solution. Dropping finishes, reflux reaction 30min, on-the-spot preparation deuterium methylmagnesium iodide (CD 3 MgI). Then, a solution of DG-2 (3.2 g, 4.85 mmol) in anhydrous tetrahydrofuran (16 ml) was added dropwise to the above reactant at 0-5°C. Stir at 0°C for 30 min, then at room temperature for 1 h. With cooling, the reaction was quenched by the careful addition of saturated aqueous ammonium chloride. Ethyl acetate was added for extraction. The organic extract was washed with saturated aqueous sodium chloride and dried over anhydrous sodium sulfate. Concentrate under reduced pressure and dry in vacuo to obtain DG-3 (quantit...
Embodiment 3
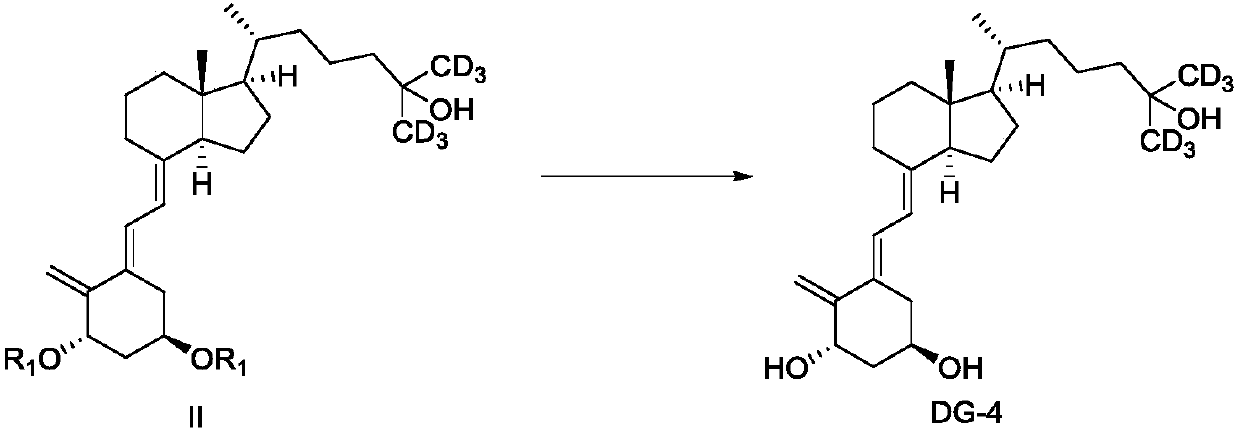
[0101] Preparation of (1α,3β,5E,7E)-9,10-cyclocholesta-5,7,10(19)-triene-1,3,25-triol (DG-4)
[0102] Under an argon atmosphere, DG-3 (according to quantitative yield, 4.85mmol) obtained in Example 2 was dissolved in anhydrous tetrahydrofuran (36ml), and a 1.0mol / L tetrahydrofuran solution of tetrabutylammonium fluoride (TBAF) was added (64ml, 64mmol), stirred at room temperature for 8h. The resulting reaction mixture was concentrated under reduced pressure, and ethyl acetate was added to the residue for extraction. The organic extract was washed with half-saturated aqueous sodium chloride and dried over anhydrous sodium sulfate. Concentration under reduced pressure gave a solid residue. The obtained crude product was purified by flash silica gel preparative chromatography (eluted with ethyl acetate / petroleum ether (volume ratio 50:50)) to obtain white solid DG-4 (1.64 g, yield 80%). 1 H NMR (400MHz, CDCl 3 )δ: 6.58(d, J=11.5Hz, 1H), 5.89(d, J=11.5Hz, 1H), 5.13(d, J=1.4Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com