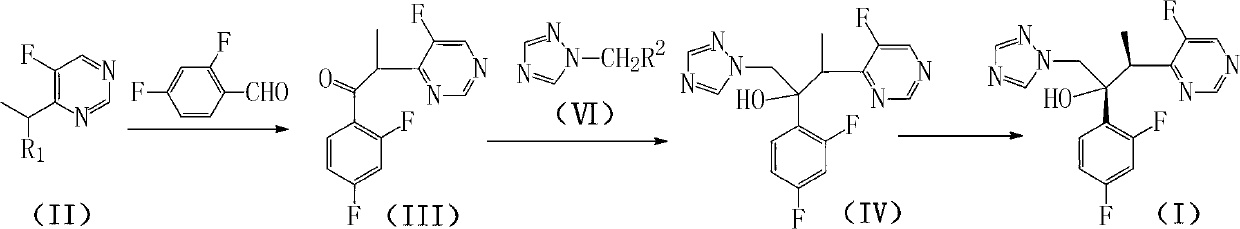
Method for preparing voriconazole and intermediate thereof
A technology of voriconazole and intermediates, applied in the field of drug synthesis, can solve the problems of harsh preparation conditions and unfavorable industrial production, and achieve the effect of short route, mild reaction conditions and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
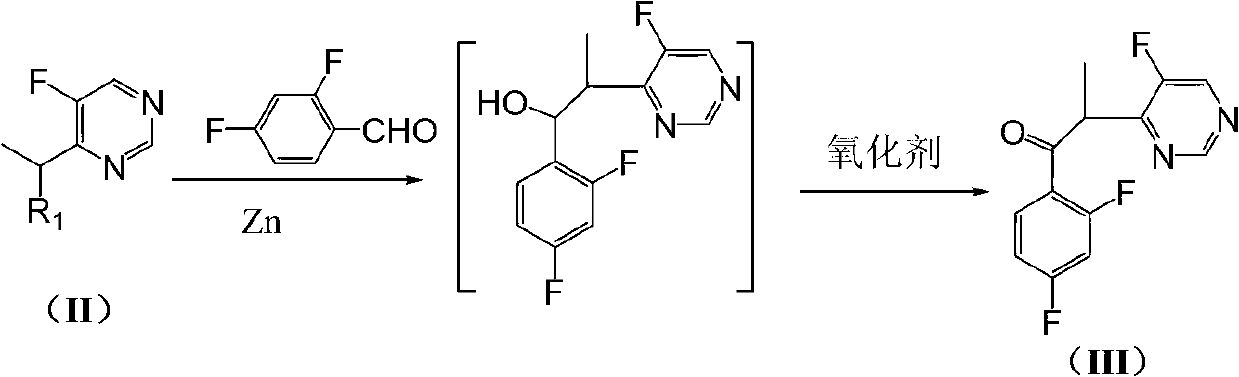
[0037] Example 1: Preparation of α-(5-fluoropyrimidin-4-yl)-2,4-difluoropropiophenone (III)
[0038]
[0039] Add 6.33g of zinc powder and 25g of THF to the three-necked flask, add 9g of iodine in 25g of THF dropwise under the protection of nitrogen at room temperature, and stir at room temperature for 30min after the dropping is complete. Add 6.4 g of 4-(1-bromoethyl)-5-fluoropyrimidine, 4.88 g of 2,4-difluorobenzaldehyde and 0.88 g of iodine in 25 g of THF dropwise with stirring in an ice bath, and control the rate of addition to keep the reaction temperature at 0°C~5°C. After dropping, the temperature was slowly raised to room temperature and stirred for 5 h, then 50 mL of saturated sodium bisulfite solution was slowly added under stirring in an ice bath, and stirred for 30 min. Filtration, the filtrate was distilled to dryness under reduced pressure, 20 mL of dichloromethane was added to the residue, the organic layer was separated, and 50 mL of saturated NaHCO 3solut...
Embodiment 2
[0040] Example 2: Preparation of α-(5-fluoropyrimidin-4-yl)-2,4-difluoropropiophenone (III)
[0041] Add 3.2Kg of zinc powder and 13Kg of THF to the reaction kettle, add 13Kg of THF solution of 5Kg of iodine dropwise under the protection of nitrogen at room temperature, and stir at room temperature for 30min after the dropping is completed. Add 3.3Kg of 4-(1-bromoethyl)-5-fluoropyrimidine, 2.44Kg of 2,4-difluorobenzaldehyde and 0.44Kg of iodine in 12KgTHF dropwise with stirring in an ice bath, and control the rate of addition to keep the reaction temperature at 0°C~10°C. After dropping, the temperature was slowly raised to room temperature and stirred for 5.5 hours. Then, 25 L of saturated sodium bisulfite solution was slowly added while stirring in an ice bath, and stirred for 30 minutes. Filtrate, recover THF from the filtrate under reduced pressure, add 2L dichloromethane to the residue, separate the organic layer, and use 25L saturated NaHCO 3 solution, 2×25L saturated N...
Embodiment 3
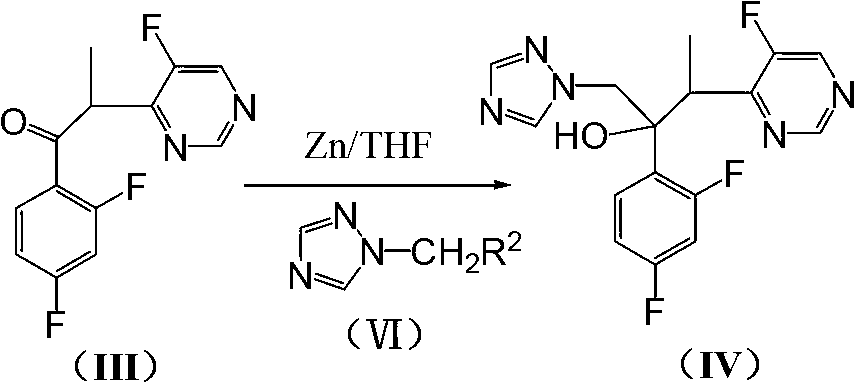
[0042] Example 3: Preparation of 1-bromomethyl-1,2,4-triazole (VI')
[0043]
[0044] Add 7.5LTHF and 630g NaH into the reaction kettle, slowly add 1050g of 1,2,4-triazole in batches under ice-bath stirring, and ice-bath stirring for 1h. Add dropwise the 500mLTHF solution of iodomethane 3300g, control the rate of addition, make the temperature of reaction remain on 5 ℃~10 ℃. After dropping, the temperature was slowly raised to room temperature and the reaction was stirred for 3h. Add 3300g of NBS and 4g of azobisisobutyronitrile into the reaction solution, and heat up to reflux for 20 hours under stirring. After cooling, the solvent was recovered under reduced pressure, 8L of dichloromethane was added to the residue, and 3×3L of saturated Na 2 CO 3 solution, 3×3L saturated NaCl solution, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was decompressed and the solvent was recovered to dryness to obtain 1686 g of brown oily compound (VI'), yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com