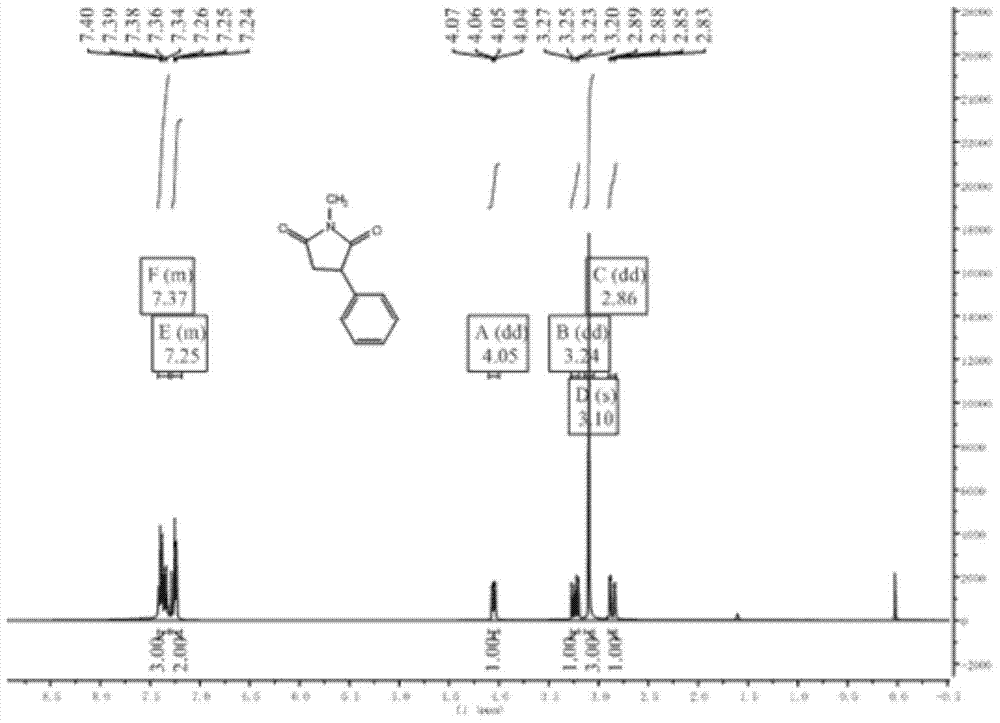
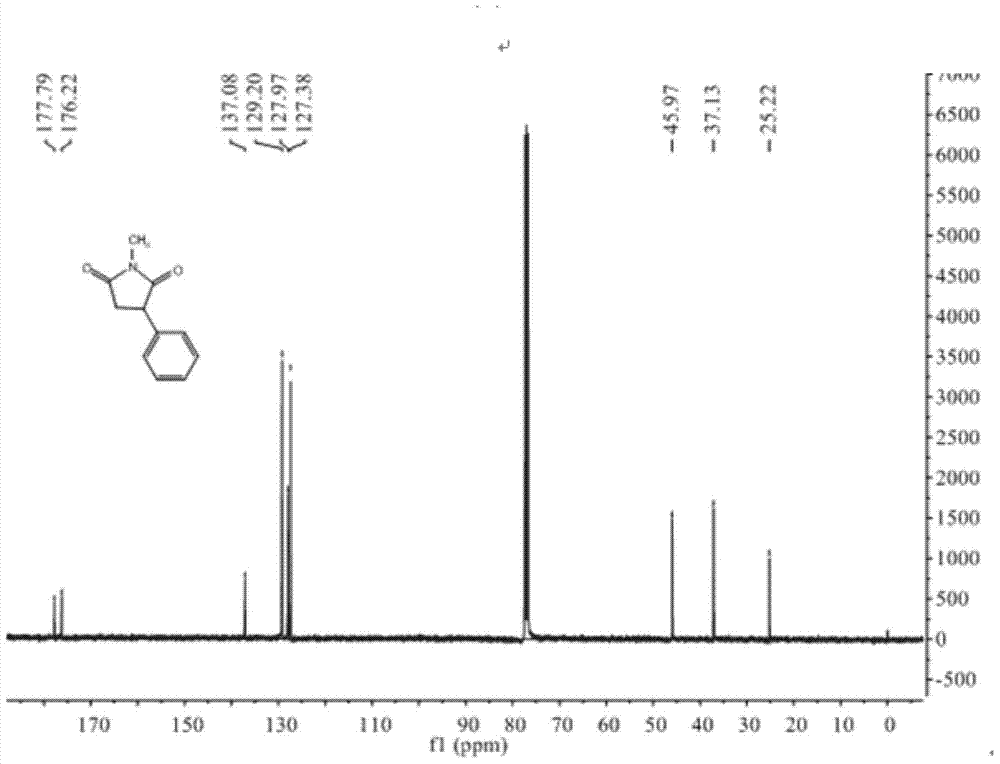
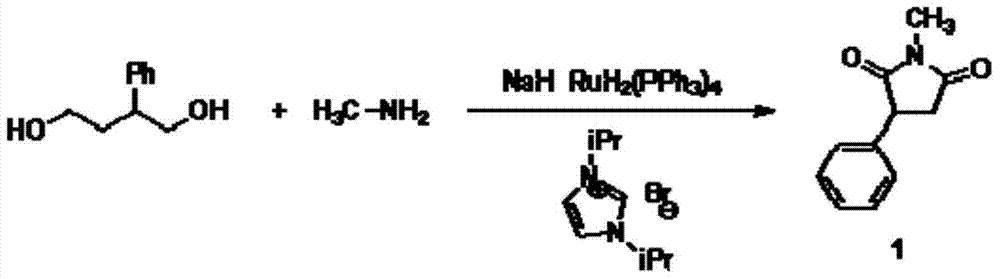
N-methyl-3 phenyl succinimide compound and preparation method thereof
A technology of phenylsuccinimide and methylmaleimide, applied in directions such as organic chemistry, can solve problems such as unfavorable industrial large-scale production, unfavorable industrialized production, NaH danger, etc., and achieves easy industrialized production and low cost , the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 46.5g (0.5mol) of aniline, 250mL of water, 220g of 40% fluoroboric acid aqueous solution, and add dropwise a solution of 36.5g (0.55mol) of sodium nitrite and 100mL of water under stirring at 0 to 5°C. After reacting for 2 hours, suction filtration was completed to obtain 80.6 g of white phenylfluoroborate diazonium salt with a yield of 84.0%, which was directly used in the next reaction.
Embodiment 2
[0039] Add 46.5g (0.5mol) of aniline, 200mL of water, and 300g of 40% fluoroboric acid aqueous solution, and add dropwise a solution of 36.5g (0.55mol) of sodium nitrite and 100mL of water at 0-5°C with stirring, and continue to stir at room temperature. After reacting for 2 hours, suction filtration was completed to obtain 85.0 g of white phenylfluoroborate diazonium salt with a yield of 88.5%, which was directly used in the next reaction.
Embodiment 3
[0041] Take 55.5g (0.5mol) of N-methylmaleimide, 565g (0.55mol) of 15% titanium trichloride aqueous solution and 140g (1.76mol) of sodium acetate, add 280mL of acetone, and stir in batches at -15°C Add 115.2 g (0.6 mol) of phenyltetrafluoroborate diazonium salt, and complete the addition in 2 hours. After the addition is completed, stir at -15°C for 1 hour, then slowly rise to room temperature and stir for 6 hours. After the reaction was completed, evaporate acetone, add ethyl acetate 1000mL for extraction, wash with water, dry the ethyl acetate layer with anhydrous sodium sulfate, evaporate the solvent, and recrystallize from ethanol to obtain 70.5g of white crystals, with a yield of about 74.6%, mp: 72~ 73°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com