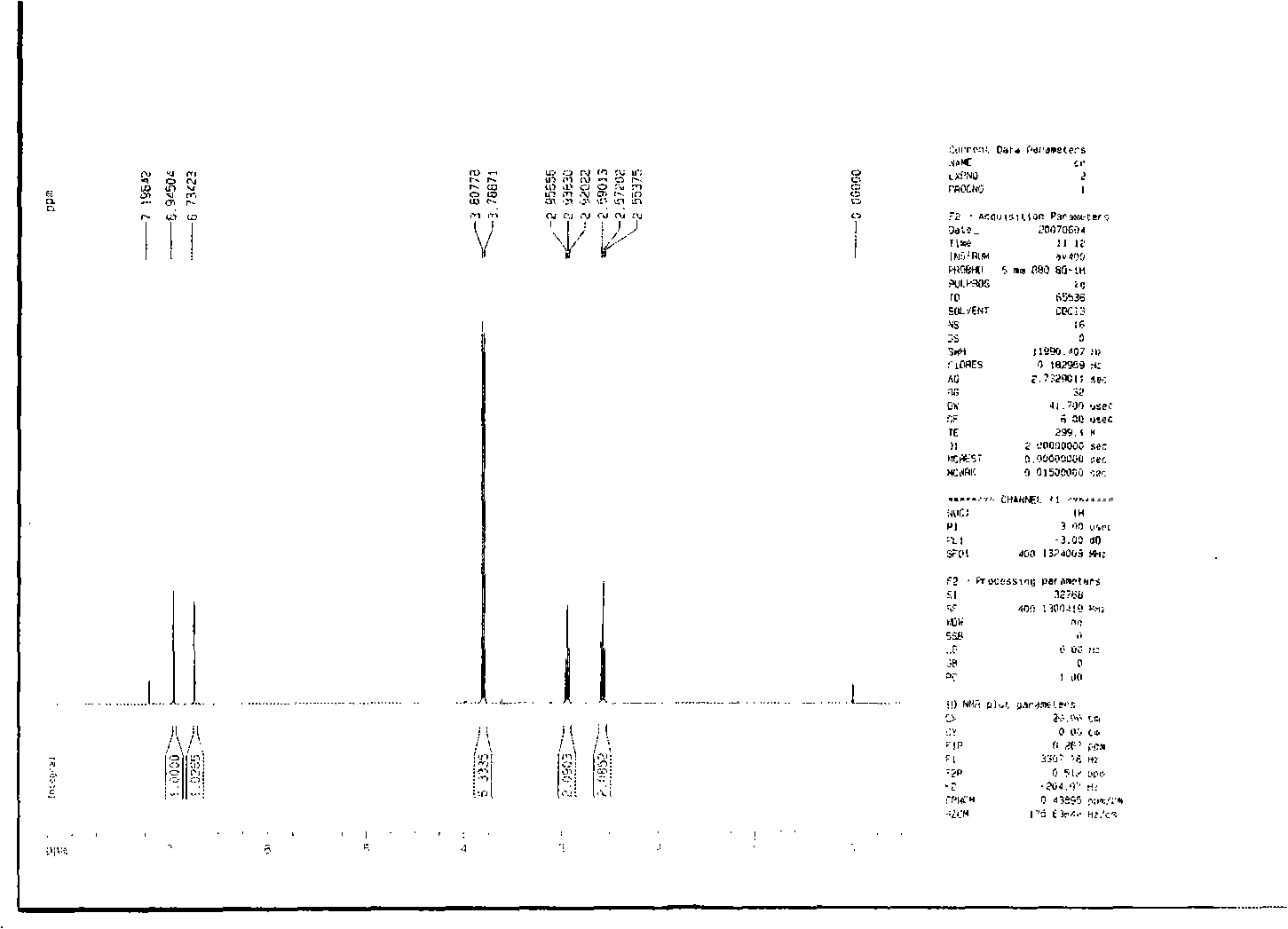
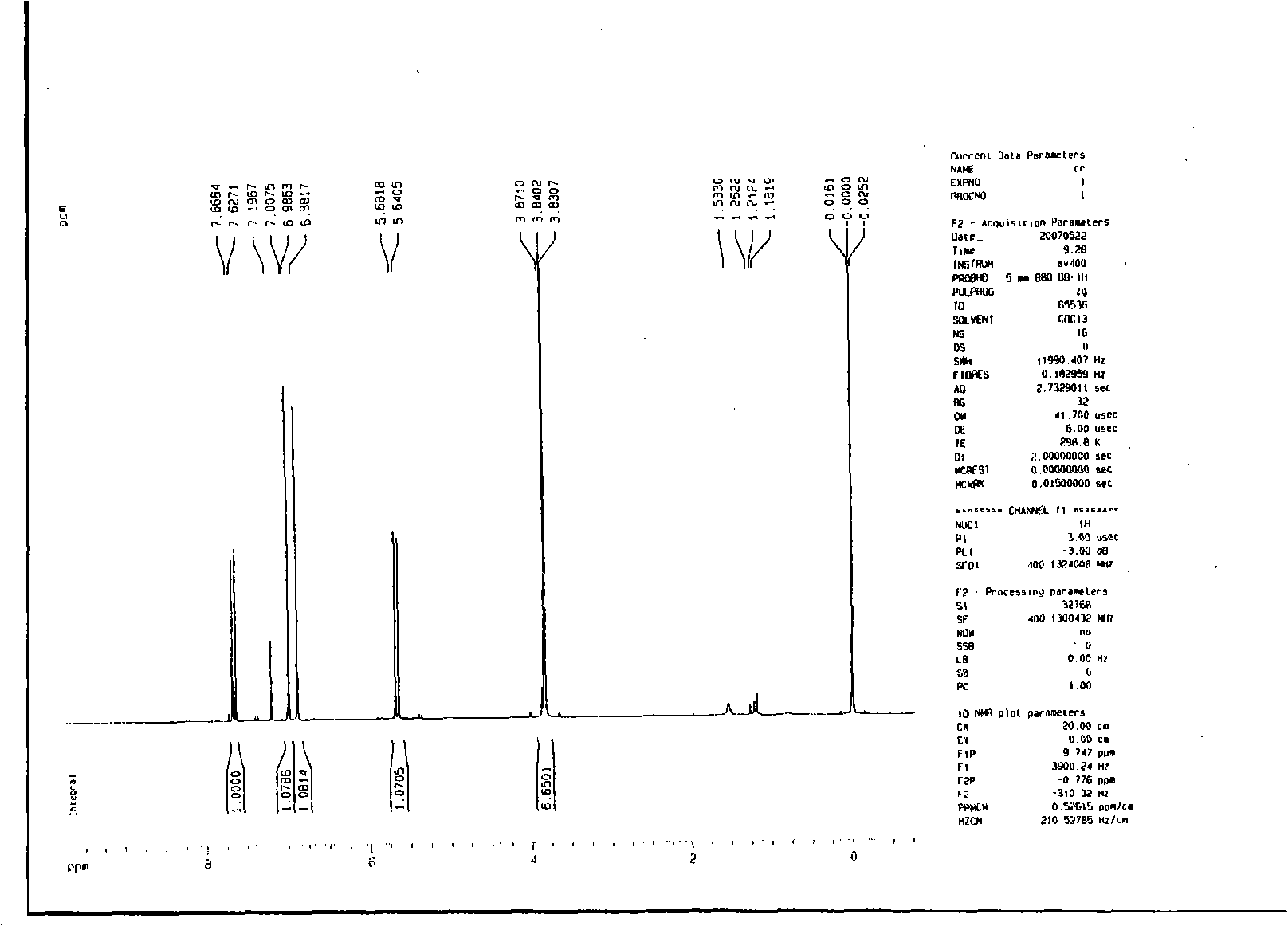
Preparation of 2-bromo-4,5-dimethoxy benzenepropanenitrile
A technology of dimethoxyphenylpropionitrile and dimethoxybenzaldehyde, applied in the field of 2-bromo-4, can solve the problems of harsh reaction conditions, long reaction route, low yield and the like, and achieves short reaction route, The effect of simple reaction operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) 2-bromo-4,5-dimethoxybenzaldehyde
[0031]
[0032] Add 100g (0.602mol) of 3,4-dimethoxybenzaldehyde and 400ml of glacial acetic acid into the reaction flask together, stir at room temperature to dissolve, slowly add 30.8ml of bromine (0.602mol) dropwise, at 20-30°C After reacting for 6 hours, 200ml of water was added to precipitate a yellow solid, which was filtered by suction, washed with water, and dried in vacuo to obtain 127.6g of white crystals of 2-bromo-4,5-dimethoxybenzaldehyde, yield 86.5%, mp: 151 ~152°C.
[0033] (2) 2-bromo-4,5-dimethoxycinnamonitrile
[0034]
[0035] Dissolve 127.6 g (0.52 mol) of 2-bromo-4,5-dimethoxybenzaldehyde in 400 ml of tetrahydrofuran and 200 ml of acetonitrile, raise the temperature to reflux, add 35 g (0.62 mol) of potassium hydroxide in batches, reflux for 10 hours, and the reaction is complete , evaporated the reaction solvent, added 200ml of water, extracted twice with ethyl acetate, anhydrous MgSO 4 Dry, distill...
Embodiment 2
[0050] (1) 2-bromo-4,5-dimethoxycinnamonitrile
[0051] Dissolve 122.0 g (0.50 mol) of 2-bromo-4,5-dimethoxybenzaldehyde in 600 ml of acetonitrile, heat up to reflux, add 24.0 g (0.60 mol) of sodium hydroxide in batches, and react at reflux for 10 hours. Add 100ml of acetonitrile, concentrate after the reaction, add 200ml of water, extract twice with ethyl acetate, anhydrous MgSO 4 After drying, the solvent was evaporated and recovered, and recrystallized from ethanol to obtain 91.4 g of 2-bromo-4,5-dimethoxycinnamonitrile, with a yield of 68.5%, and mp: 147-148°C.
[0052] (2) 2-Bromo-4,5-dimethoxyphenylpropionitrile
[0053] Dissolve 120.0g (0.45mol) of 2-bromo-4,5-dimethoxycinnamonitrile in 100ml of pyridine and 400ml of methanol, add 48.6g (0.90mol) of potassium borohydride in batches, and slowly raise the temperature and reflux for 24 hours , after the reaction was completed and cooled, the unreacted potassium borohydride was decomposed with 10% hydrochloric acid, extra...
Embodiment 3
[0055] (1) 2-bromo-4,5-dimethoxyphenylpropionitrile
[0056] Dissolve 120.0 g (0.45 mol) of 2-bromo-4,5-dimethoxycinnamonitrile in 800 ml of methanol, add 20 g of 10% palladium carbon in batches, and pass through hydrogen for reduction reaction for 12 hours. After the reaction is complete, filter off Palladium carbon, the solvent was distilled off and recovered, and recrystallized from ethanol to obtain 102.4 g of 2-bromo-4,5-dimethoxyphenylpropionitrile as white crystals, with a yield of 84.6%, mp: 76-78°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com