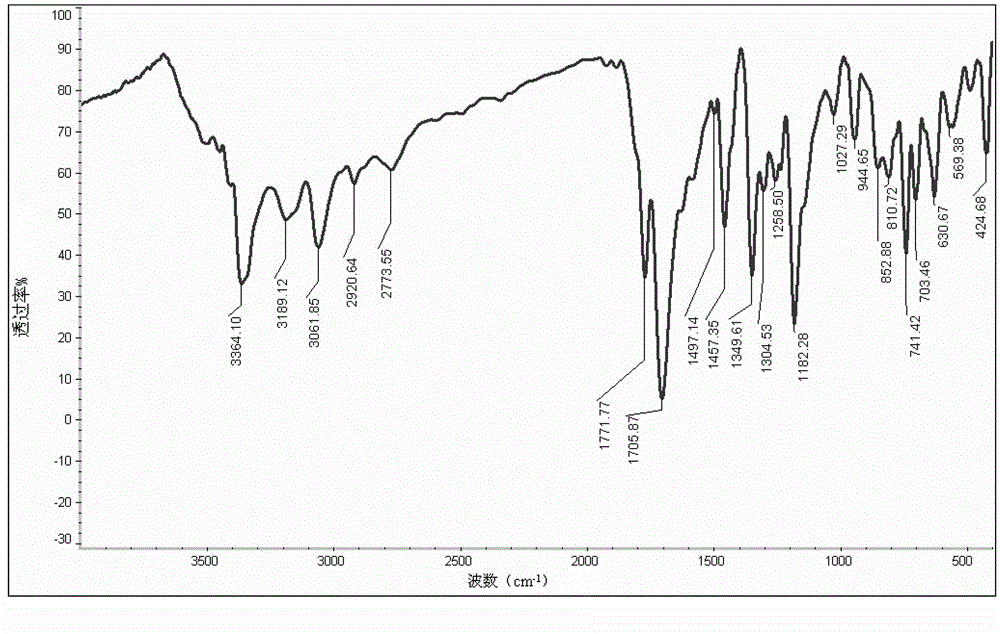
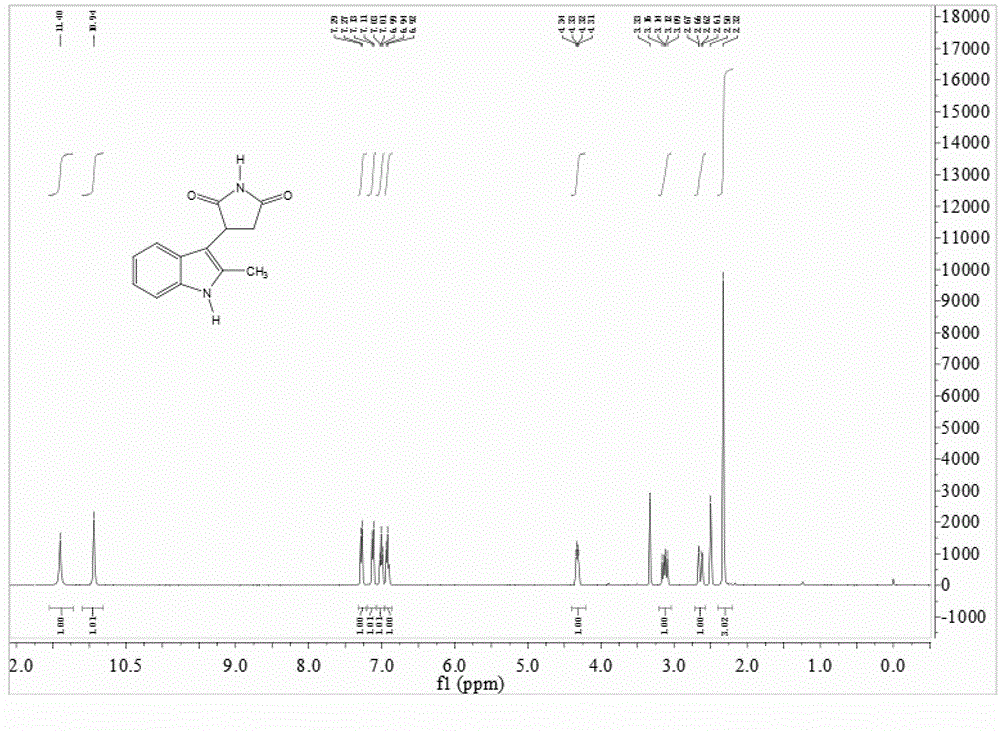
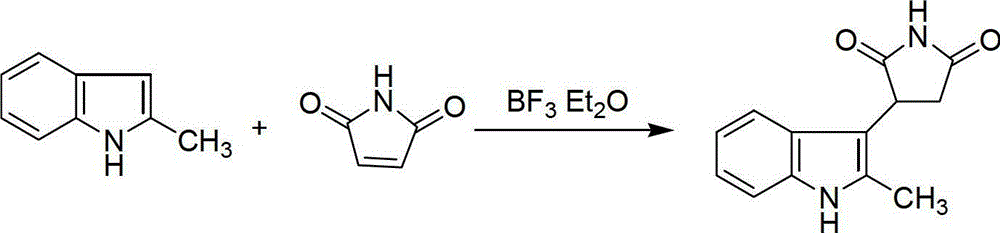Preparation method of 3-(2-methylindolyl-3-)pyrryl-2,5-dione
A technology of methyl indole and diketone, which is applied in the field of preparation of pharmaceutical synthesis intermediates, can solve the problems of long reaction time, long reaction route and high cost, and achieves the effects of short reaction route, low cost and reduction of three-waste treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 26.2g (0.2mol) of 2-methylindole, 19.4g (0.2mol) of maleimide and 5.0mL (0.04mol) of boron trifluoride ether into a 500mL round bottom flask, add 1,2- Dichloroethane 300mL, stirred and refluxed for 5h, after the reaction was completed, distilled and recovered 1,2-dichloroethane, added 300mL of water, stirred for 10 minutes, suction filtered, washed with water, and the obtained solid was recrystallized with 95% ethanol to obtain a white solid 39.7 g, yield 87%, mp: 198~200°C.
Embodiment 2
[0032] Take 26.2g (0.2mol) of 2-methylindole, 19.4g (0.2mol) of maleimide and 5.0mL (0.04mol) of boron trifluoride ether and add them to a 500mL round bottom flask, add 300mL of chloroform, and heat Stir and reflux the reaction for 24 hours. After the reaction is complete, distill off the chloroform and recover it. Add 300 mL of water, stir for 35 minutes, filter with suction, wash with water, and recrystallize the obtained solid with 95% ethanol to obtain 34.7 g of white solid, yield 76%, mp: 197~200 ℃.
Embodiment 3
[0034] Take 26.2g (0.2mol) of 2-methindole, 19.4g (0.2mol) of maleimide and 5.0mL (0.04mol) of boron trifluoride ether into a 500mL round bottom flask, add 1,2-di Add 300 mL of ethyl chloride, heat to 70°C and stir for 14 hours. After the reaction is complete, distill and recover 1,2-dichloroethane, add 300 mL of water, stir for 60 minutes, filter with suction, and wash with water. The obtained solid was recrystallized with 95% ethanol to obtain 33.3 g of white solid, yield 73%, mp: 196~199°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com