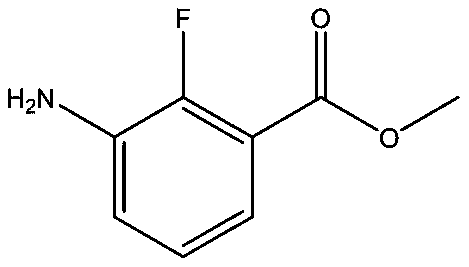
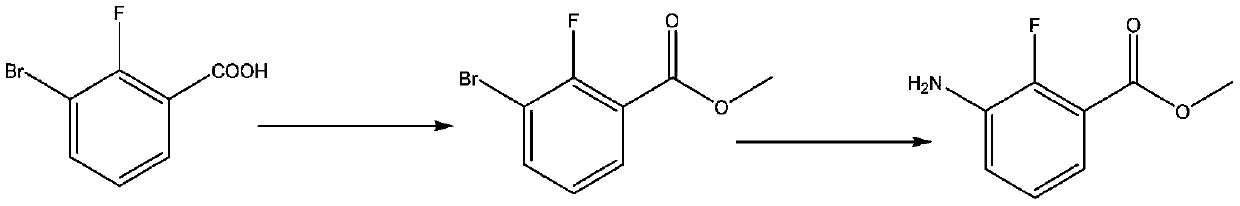
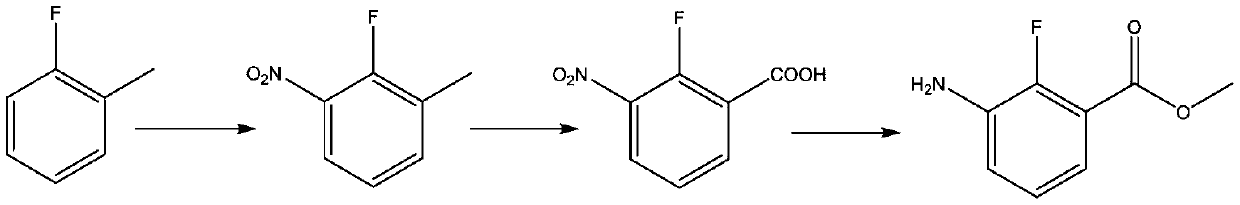
Synthesis method of anti-cancer drug intermediate methyl 2-fluoro-3-aminobenzoate
A technology of methyl aminobenzoate and methyl nitrobenzoate is applied in the field of synthesis of new anticancer drug intermediate 2-fluoro-3-aminobenzoic acid methyl ester, and can solve the problems such as difficulty in obtaining raw materials, high environmental protection pressure, etc. problem, to achieve the effect of increased reaction continuity and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Under nitrogen protection, 100g (0.5235mol) of 2,6-dichlorobenzoic acid was added in batches to 223g (2.093mol, 4eq) of 92% concentrated sulfuric acid, and the pre-configured 68 Mixed acid of 72g of concentrated nitric acid and 55g of 92% concentrated sulfuric acid, after the dropwise addition, the temperature was raised to 30°C for 0.5-1 hour reaction, sampling HPLC was performed to detect that the raw material was 1 HNMR (400MHz, MeOH-d 4 ):δ=7.98(d,1H),7.66(d,1H).
Embodiment 2
[0038] Under the protection of nitrogen, mix 100g (0.5235mol, 1eq) of 2,6-dichlorobenzoic acid and 70g (0.657mol, 1.26eq) of 92% concentrated sulfuric acid, and add the pre-configured Mixed acid of 36g of 95% fuming nitric acid and 38g of 92% concentrated sulfuric acid, react at room temperature for 5 hours after the dropwise addition, sample HPLC to detect the raw material <0.2%, cool to 0-10°C, add 8 times the volume of dichloromethane each time to carry out Extraction, a total of two extractions, combined the organic phases, adding water at 0-10 ° C to wash until the pH of the aqueous phase = 2-3, and concentrating the organic phases under reduced pressure to stagnant liquid to obtain the intermediate 2,6-dichloro-3-nitrate Base benzoic acid 116.8g, HPLC detection chemical purity 98.3%, yield 94.5%.
[0039] The second step: the synthesis of methyl 2,6-dichloro-3-nitrobenzoate
Embodiment 3
[0041] Dissolve 117.7 g of 2,6-dichloro-3-nitrobenzoic acid in 259 g of methanol, add 7.6 g of concentrated sulfuric acid, heat up to reflux, and react for 5 hours, sample HPLC to detect that the raw material is 1 HNMR (400MHz, DMSO-d 6 ):8.28(s,1H),7.52(s,1H),3.89(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com