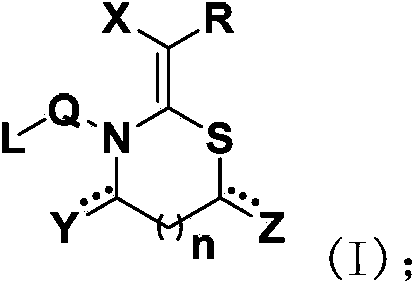
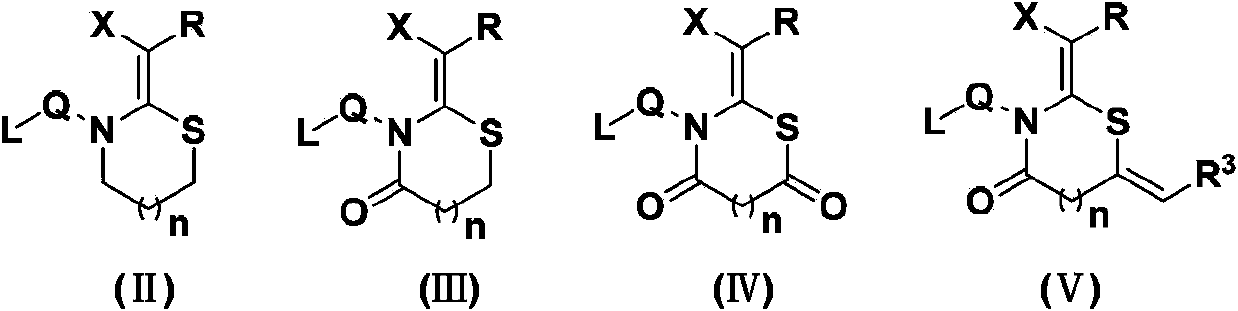
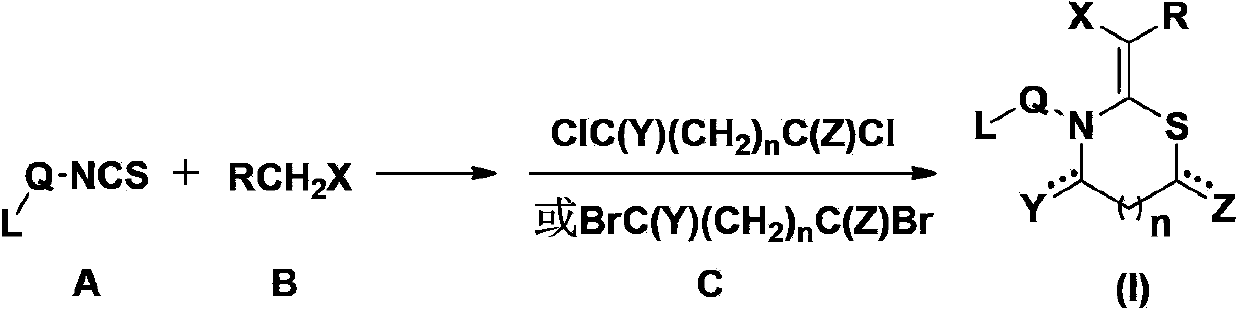
N, S-containing heterocyclic compound with nematicidal activity, preparation method and application thereof
A compound and heterocyclic technology, which is applied in the field of pesticides, can solve the problems of secondary infection by pathogens, plundering of host plants, and increasing difficulty in nematode control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0117] The preparation method of the compound of the present invention
[0118] The compound represented by the general formula (I) of the present invention can be prepared by the following method, but the conditions of the method, such as the amount of reactant, solvent, base, compound used, reaction temperature, reaction time required, etc. are not limited to the following explanations . The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0119] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent at a reaction temperature of 0-60°C (preferably 20-40°C). The reaction time is usually 2-24 hours, preferably 4-12 hours.
[0120] The base used in the reaction includes (but is not limited t...
Embodiment 1
[0146] Example 12-(1-cyano-1-methyl carboxylate)-3-N-(2-fluorophenyl)-1,3-thiazol-4-one
[0147] 1.1 Preparation of 2-fluorophenylisothiocyanate
[0148]
[0149] Add 1.11g (10mmol) of 2-fluoroaniline and 3.36g of triethylenediamine (30mmol) into 15ml of acetone, stir to dissolve them, add dropwise 45ml of carbon disulfide, and stir the reaction at room temperature. The reaction was tracked by TLC. After the reaction of 2-fluoroaniline was complete, it was filtered and dried to obtain a powdery solid. It was added in 30ml chloroform, stirred to make it into a suspension, down to 0°C, slowly added dropwise 10ml chloroform solution of 0.99g (3.34mmol) bis(trichloromethyl)carbonate (BTC), added, The temperature was raised to room temperature for reaction, followed by TLC. After the reaction, the insoluble matter was removed by filtration, and the solvent was evaporated under reduced pressure to obtain 1.23 g of a yellow solid, which was directly used in the next step without...
Embodiment 2
[0153] Example 22-(1-cyano-1-methyl carboxylate)-3-N-(4-nitrophenyl)-1,3-thiazol-4-one
[0154]
[0155] The preparation method of 4-nitrophenyl isothiocyanate is similar to step 1.1 of Example 1, except that 4-nitroaniline is used instead of 2-fluoroaniline.
[0156] Add 0.56g (10mmol) potassium hydroxide to 20ml DMF, stir to make it into a suspension, add 0.5 g (5mmol) methyl cyanoacetate, be down to 0 ℃, dropwise add the 5ml DMF solution of 0.9g (5mmol) 4-nitrophenyl isothiocyanate, add, be warming up to room temperature reaction. The reaction was tracked by TLC. After the reaction of 4-nitrophenylisothiocyanate was complete, the temperature was lowered to 0°C, and 0.565 g (5 mmol) of chloroacetyl chloride was slowly added dropwise. After the addition was completed, the temperature was raised to room temperature for 8 hours. The reaction solution was poured into ice water, a large amount of yellow solids were precipitated, filtered, and recrystallized from ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com