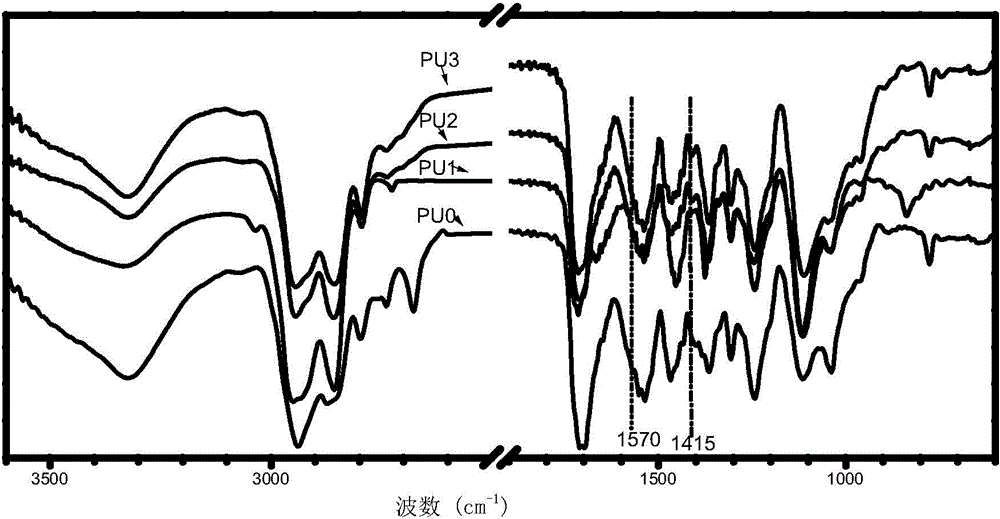
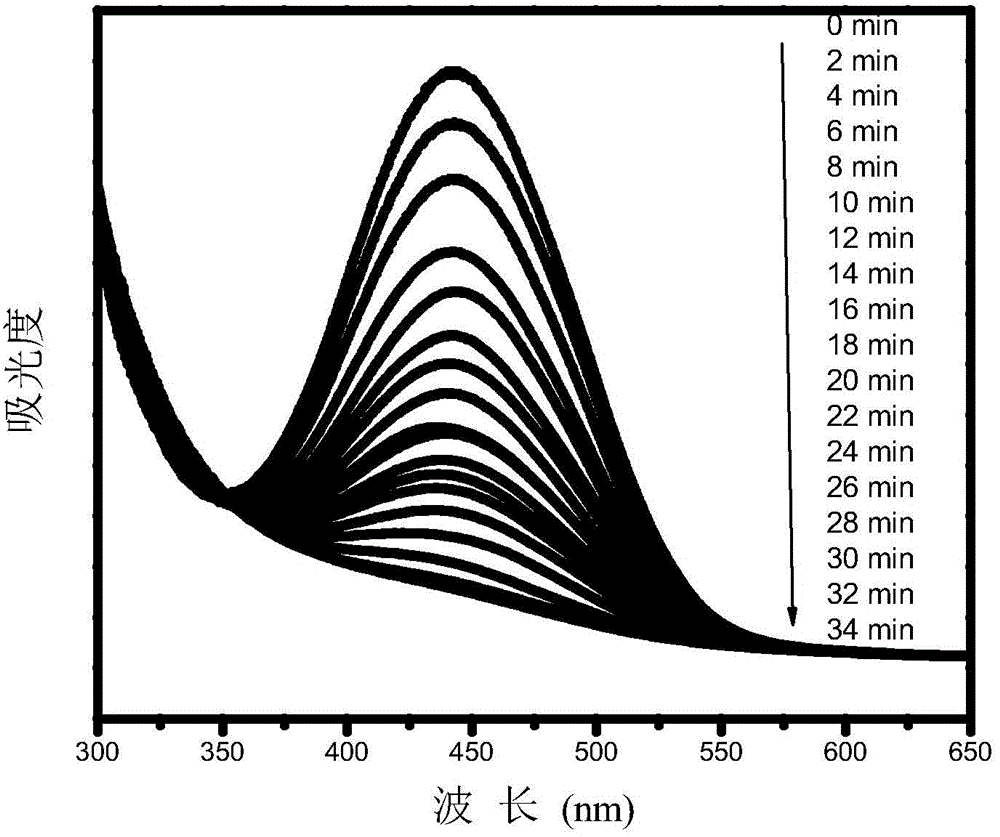
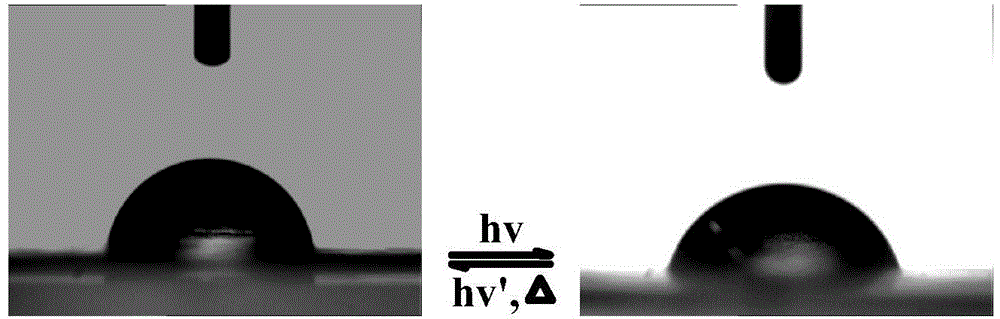
Preparation method of aqueous polyurethane based on anionic azo hydrophilic chain extender
A technology of hydrophilic chain extender and water-based polyurethane, which is applied in the preparation of water-based polyurethane, and in the field of water-based polyurethane preparation based on anionic azo hydrophilic chain extender, which can solve the problems affecting the cis-trans isomerization efficiency of azo groups, Poor material migration resistance, limited doping concentration and other problems, to achieve the effect of long-term maintenance of azo functional properties, good acid and alkali resistance, and reduced procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 50.00g PBA (M n =3200) into a 500mL three-necked flask, dehydrated at 110°C for 1 hour, then cooled to 55°C, took 11.10g of TDI and added it to the three-necked flask, stirred and reacted at 80°C for 3 hours, then added 9.60g of sulfonic acid type azophile Water chain extender, 1.50g DEG, 0.01g DBTDL and 45.00g methyl ethyl ketone, stirred and reacted at a constant temperature of 70°C for 3 hours, then cooled to 30°C, transferred the reaction solution into a high-speed shear disperser, at 3000 rpm Add 4.36g TEA under low pressure, add 150g water after reacting for 5 minutes, add 0.35g EDA to react for 30 minutes after stirring for 1 minute, transfer the reaction solution to a rotary evaporator, remove butanone under 45°C and 0.01MPa vacuum conditions, A sulfonic acid type azo aqueous polyurethane emulsion is obtained.
[0040] If other conditions of the present embodiment remain unchanged, and PBA is replaced by PCL, PCDL, PTMG or PPG respectively, a stable colored wat...
Embodiment 2
[0042] 160.00g PPG (M n =2000) into a 1000mL three-necked flask, dehydrated at 120°C for 1 hour, then cooled to 60°C, took 62.10g of IPDI and added it to the three-necked flask, stirred and reacted at 90°C for 3 hours, then added 17.20g of carboxylic acid type azophile Water chain extender, 6.90g BDO, 0.01g DBTDL and 120.00g acetone, stirred and reacted at a constant temperature of 75°C for 3 hours, then cooled down to 30°C, transferred the reaction solution into a high-speed shear disperser, under the condition of 3000 rpm Add 14.66g TEA, add 580g water after reacting for 5 minutes, add 0.90g IPDA to react for 30 minutes after stirring for 1 minute, transfer the reaction solution to a rotary evaporator, remove acetone under 40°C and 0.01MPa vacuum conditions to obtain carboxyl Acid type azo water-based polyurethane emulsion.
[0043] If other conditions of this embodiment remain unchanged, and BDO is replaced by HDO, EG or DEG, a stable colored water-based polyurethane emuls...
Embodiment 3
[0046] 80.00g PTMG (M n =2000) into a 500mL three-necked flask, dehydrated at 120°C for 1 hour, then cooled to 60°C, took 35.10g of IPDI and added it to the three-necked flask, stirred and reacted at 90°C for 3 hours, then added 2.30g of carboxylic acid type azophile Water chain extender, 5.5g dimethylolpropionic acid, 6.90g HDO, 0.01g DBTDL and 70.00g methyl ethyl ketone, stirred and reacted at 75°C for 3 hours at a constant temperature, then cooled to 30°C, and the reaction solution was transferred to high-speed shear dispersion machine, add 5.39g TEA under the condition of 3000 rev / min, add 290g water after reacting for 5 minutes, and then transfer to rotary evaporator after stirring for 1 minute, remove methyl ethyl ketone under 45°C and 0.01MPa vacuum condition to obtain carboxy Acid / sulfonic acid hybrid pigmented aqueous polyurethane emulsion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com