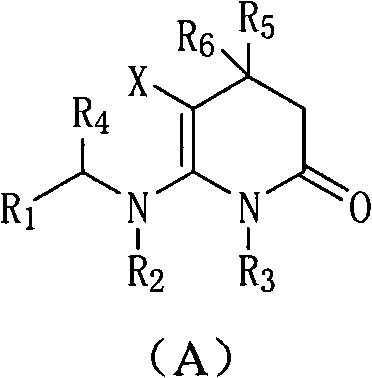
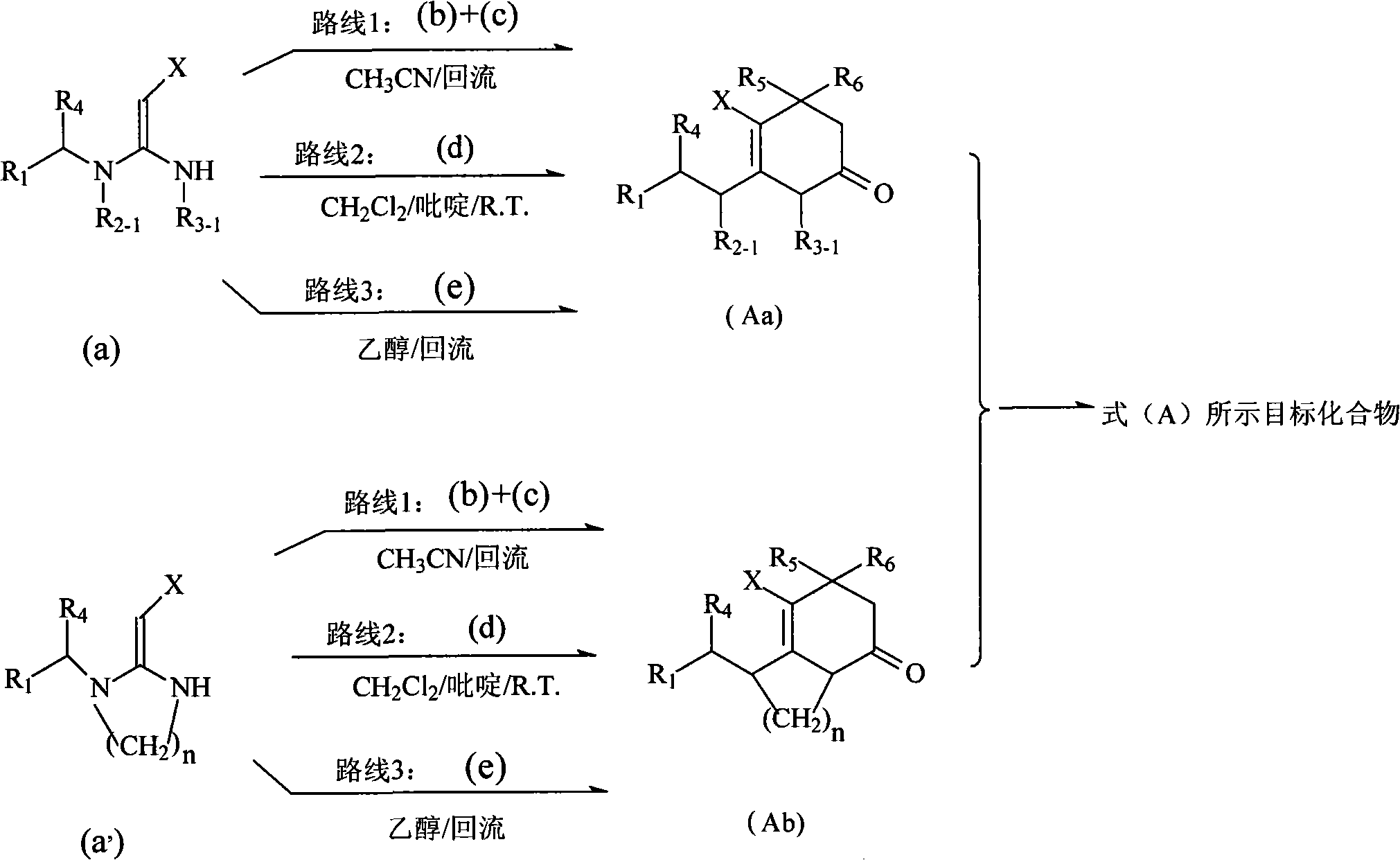
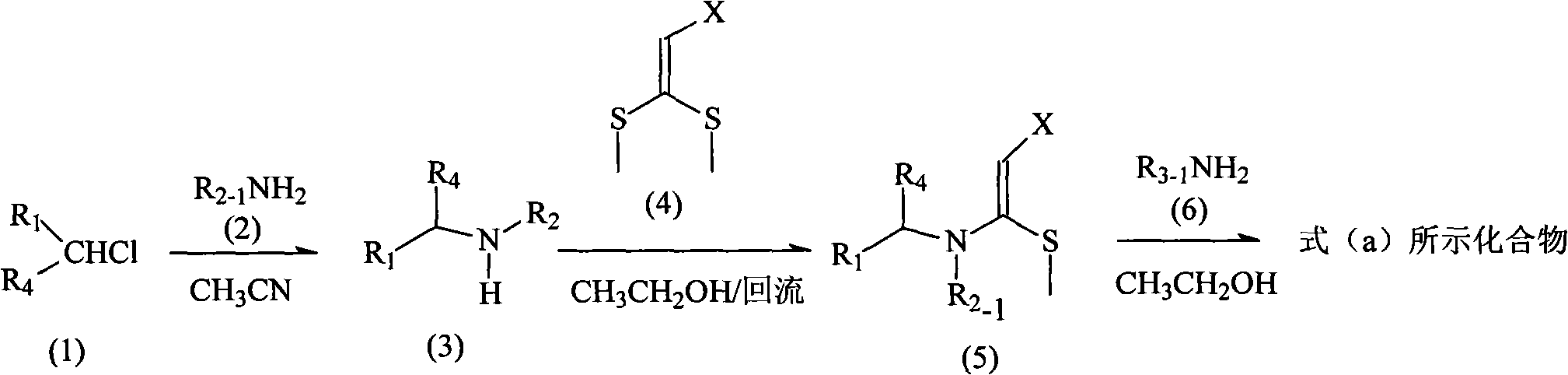
3,4-dihydropyridine-2-ketone heterocyclic compound and application thereof
A compound, six-membered heterocycle technology, applied in the field of nicotinic compounds, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Synthesis of compound (Aa-1):
[0093]
[0094] Under nitrogen protection, add 94.38mg (0.39mmol) compound (a-1), 68mg (0.47mmol) compound (b), 1mL anhydrous acetonitrile, 0.05mL (0.47mmol) compound (c) to a 10mL Schlenk tube successively -1), 0.005 mL (0.04 mmol) triethylamine. The temperature of the reaction solution was slowly raised to 80°C-90°C under stirring, and the reaction was stopped after reflux for 4 hours. A large amount of solvent was removed by a rotary evaporator, and the reaction solution was concentrated and then directly separated by column chromatography (eluent: ethyl acetate) to obtain 100 mg of light yellow solid powder. The yield was 69%. Melting point: 213°C-214°C.
[0095] Note: All reagents, reaction vials and condenser tubes in this reaction were dried, and benzaldehyde was redistilled.
[0096] 1H NMR (400MHz, DMSO): δ8.95(s, 1H), 8.17(d, J=2.8Hz, 1H), 7.29-7.26(m, 3H), 7.12(d, J=8.0Hz, 1H), 7.10-7.08(m, 2H), 4.95(t, J=4.4Hz, 1H), 4...
Embodiment 2
[0099] Synthesis of compound (Aa-2):
[0100]
[0101] Under nitrogen protection, add 99.84mg (0.39mmol) compound (a-1), 68mg (0.47mmol) compound (b), 1mL anhydrous acetonitrile, 0.05mL (0.47mmol) compound (c) to a 10mL Schlenk tube successively -2), 0.005 mL (0.04 mmol) triethylamine. The temperature of the reaction solution was slowly raised to 80°C-90°C under stirring, and the reaction was stopped after reflux for 4 hours. A large amount of solvent was removed by a rotary evaporator, and the reaction solution was concentrated and separated by column chromatography (eluent: ethyl acetate) to obtain 96 mg of light yellow solid powder. The yield is 66%. Melting point: 83°C-84°C.
[0102] Note: All reagents, reaction flasks and condenser tubes in this reaction were dried, and furfural was redistilled.
[0103] 1 H NMR (400MHz, DMSO): δ8.95(s, 1H), 8.18(d, J=2.8Hz, 1H), 7.74(d, J=7.2, 1H), 7.21(d, J=8.0Hz, 1H ), 7.10-7.08(m, 2H), 4.89(t, J=3.2Hz, 1H), 4.83(d, J=2.4Hz, 2...
Embodiment 3
[0106] Synthesis of compound (Aa-3):
[0107]
[0108] Under nitrogen protection, add 86.58mg (0.39mmol) compound (a-2), 68mg (0.47mmol) compound (b), 1mL anhydrous acetonitrile, 61.57mg (0.47mmol) compound (c) to a 10mL Schlenk tube successively -3), 0.005 mL (0.04 mmol) triethylamine. The temperature of the reaction solution was slowly raised to 80°C-90°C under stirring, and the reaction was stopped after reflux for 4 hours. A large amount of solvent was removed by a rotary evaporator, and the reaction solution was concentrated and separated by column chromatography (eluent: ethyl acetate) to obtain 112 mg of light yellow solid powder. The yield was 76%. Melting point: 203°C-204°C.
[0109] Note: All reagents, reaction vials and condenser tubes for this reaction were dried.
[0110] 1 H NMR (400MHz, DMSO): δ8.98(s, 1H), 8.28(d, J=2.8Hz, 1H), 7.64(d, J=7.2, 1H), 7.29-7.26(m, 3H), 7.12 (d, J=8.0Hz, 1H), 5.12(t, J=3.2Hz, 1H), 4.86(d, J=2.8Hz, 2H), 3.93-3.83(m, 2H), 2.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com