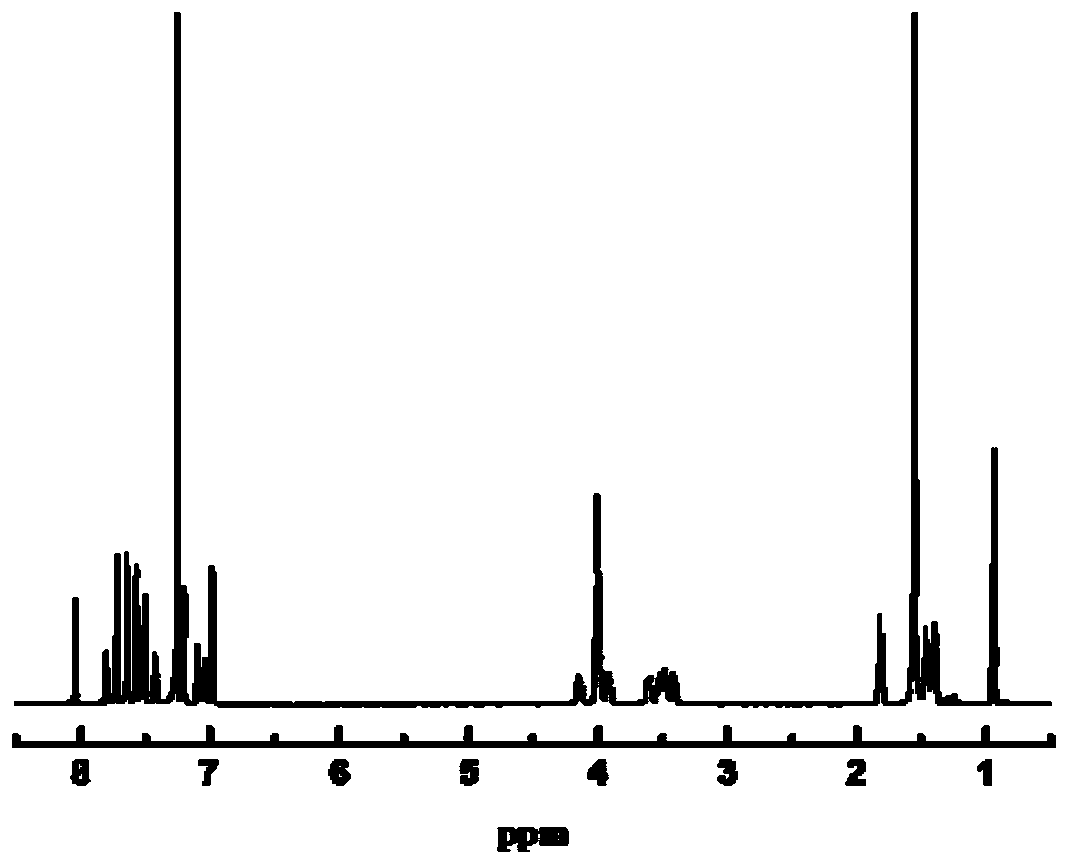
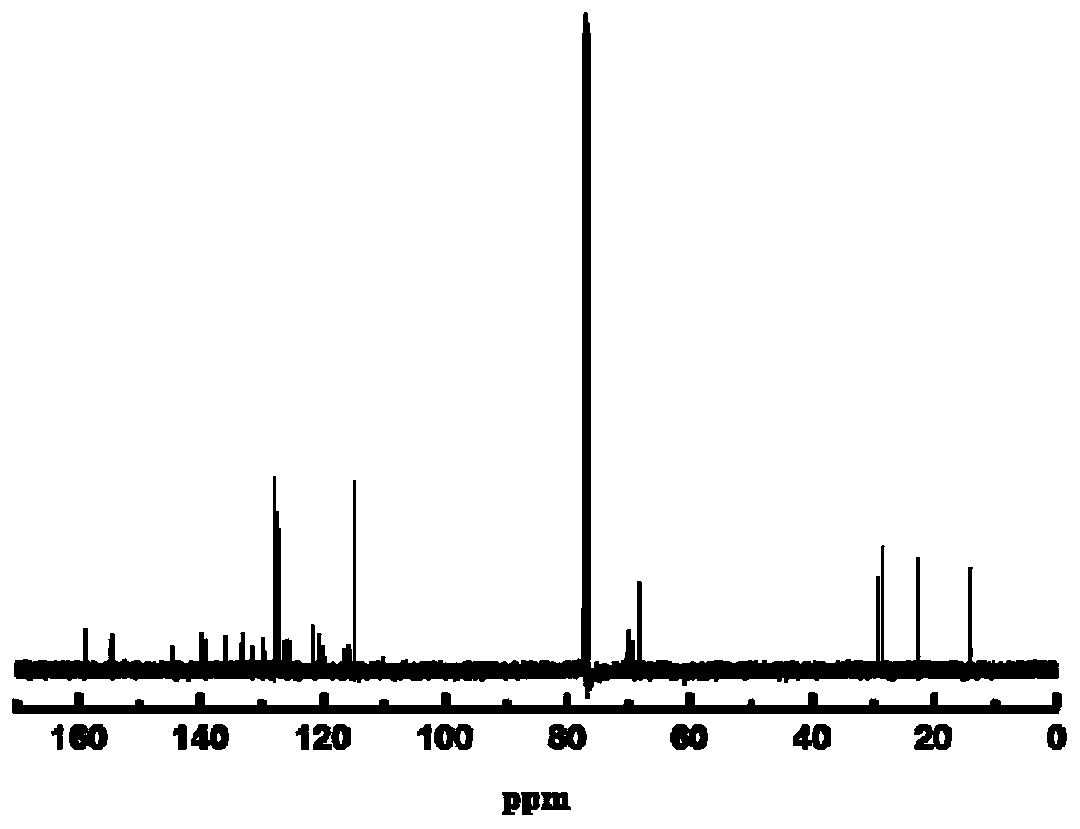
Dinaphthalene azobenzene ring-type photosensitive chiral molecule and preparation method and application thereof
A technology of binaphthylazobenzene and chiral molecules, which is applied in the field of preparation of light-responsive materials and achieves the effect of good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The specific synthesis steps of R-binaphthazobenzene photosensitive cyclic chiral molecules are as follows:
[0036] 1.1 Preparation of intermediate 1
[0037] Dissolve (R)-6,6'dibromo-1,1'-bi-2-naphthol (1.0g, 2.3mmol), potassium carbonate (1.1g, 7.9mmol), potassium iodide (0.1g, 0.7mmol) In N,N-dimethylformamide, after stirring the reaction at 100°C for 20min, add 2-(2-chloroethoxy)ethanol (1.0g, 7.9mmol) and continue the reaction at 100°C 24h. After the reaction was completed and cooled to room temperature, the reaction solution was poured into ice water for dilution, extracted three times with ethyl acetate and saturated saline, dried over anhydrous sodium sulfate and subjected to silica gel column chromatography to obtain intermediate 1, whose structural formula is as follows:
[0038]
[0039] 1.2 Preparation of Intermediate 2
[0040] Weigh the intermediate 1 (1g, 1.6mmol) and 4-pentyloxybiphenylboronic acid (1.1g, 3.84mmol) and dissolve it in 1,4-dioxane, ...
Embodiment 2
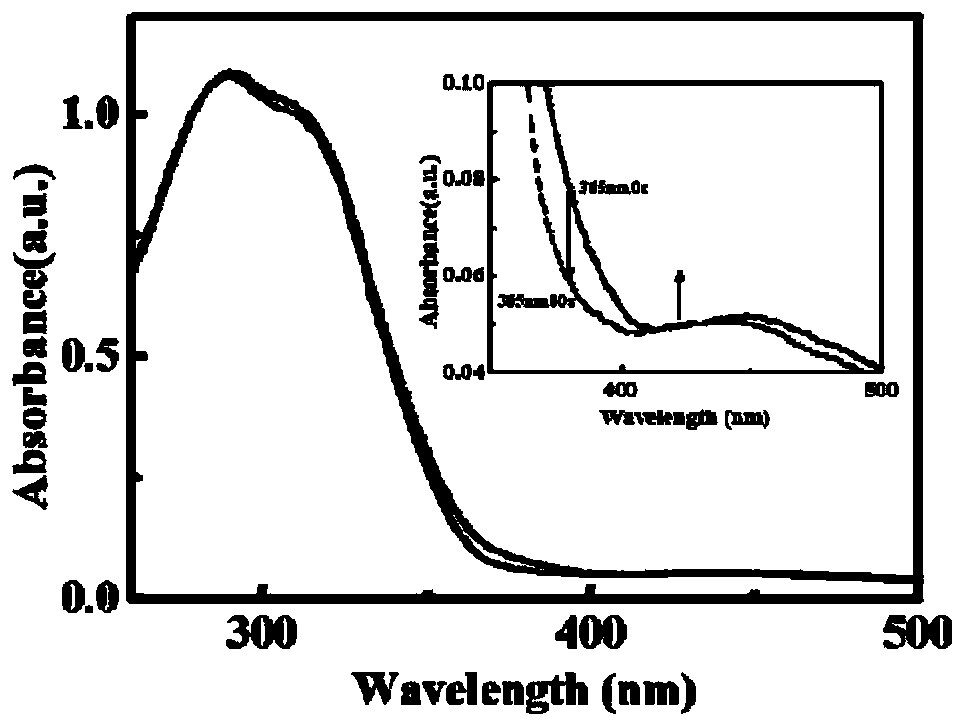
[0051] Changes of Twisting Force Constant of Cholesteric Liquid Crystals
[0052] Add 0.002 g of the binaphthazobenzene photosensitive cyclic chiral molecule to 0.100 g of liquid crystal, heat to the clearing point for 2 hours, cool to room temperature, and then slowly pour into a wedge cell (tanθ=0.01). After standing for a period of time, the change of the thread pitch was measured by polarizing microscope under the illumination of 365nm and 440nm respectively, and the corresponding thread pitch changed from 17.5 μm to 20.7 μm and finally to 19.5 μm.
Embodiment 3
[0054] Compatibility determination
[0055] The Teas solubility parameters of the binaphthazobenzene photosensitive cyclic chiral molecule, the Azo-o-Bi chiral agent molecule and the liquid crystal host E7 molecule were calculated respectively, and the differences between the two kinds of chiral agent molecules and the liquid crystal host were compared. Teas parameter difference (Δf), in general, the compatibility between chiral agent molecules and liquid crystal molecules decreases with the increase of Δf value. By calculation, the Δf value between the Azo-o-Bi chiral agent molecule and the liquid crystal host molecule is 0.17, and the Δf value between the binaphthazobenzene photosensitive cyclic chiral molecule and the liquid crystal host molecule is reduced to 0.15, improving the The compatibility with the liquid crystal host molecules is improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com