A method of regulating supramolecular polymerization
A supramolecular polymer and supramolecular technology, applied in the field of supramolecular chemistry, achieves the effects of strong responsiveness, simple and fast method, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
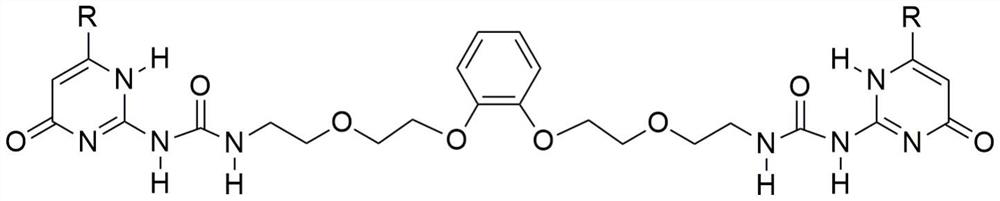
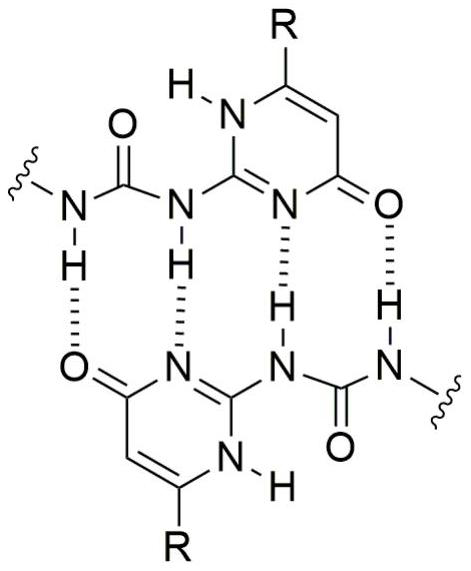
[0031] Add 0.5 ml of deuterated chloroform and deuterated acetonitrile at a volume ratio of 1:1 to the NMR tube, add 48 mg of isoheptyl-substituted monomer, dissolve it by ultrasonic, and prepare a 128 mM solution. The proton nuclear magnetic resonance spectrum test was carried out, wherein the NH characteristic peaks of ureidopyrimidinone represented polymers were 13.07ppm, 11.86ppm and 10.17ppm respectively. Add 1 equivalent of KPF 6 Afterwards, the H NMR spectrum test was carried out again. Due to the formation of cyclic dimers, the dimerization planes of ureido pyrimidone were parallel to each other, resulting in shielding, and the peaks of NH moved to high fields, moving to 13.05ppm, 11.72ppm and 9.74ppm respectively. .
Embodiment 2
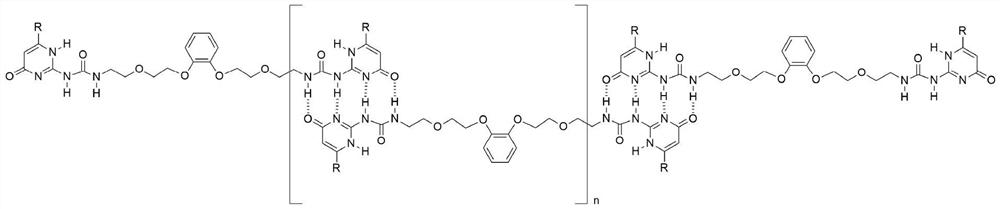
[0033] KPF 6 Viscosity tests were performed on the isoheptyl-substituted monomers and independent monomers, respectively. As the concentration increases, the independent monomer first maintains a growth trend with a slope of 1, and after approaching 10mM, it begins to grow with a slope of 1.7, indicating that polymerization has occurred; while adding KPF 6 As the concentration increases, the monomer always maintains a growth trend with a slope of 1, indicating that it always maintains a dimer form with a small molecular weight.
Embodiment 3
[0035] Add 0.5 ml of deuterated chloroform and deuterated acetonitrile at a volume ratio of 1:1 to the NMR tube, add 48 mg of isoheptyl-substituted monomer, dissolve it by ultrasonic, and prepare a 128 mM solution. The proton nuclear magnetic resonance spectrum test was carried out, wherein the NH characteristic peaks of ureidopyrimidinone represented polymers were 13.07ppm, 11.86ppm and 10.17ppm respectively. Add 1 equivalent of KPF 6 Afterwards, the H NMR spectrum test was carried out again. Due to the formation of cyclic dimers, the dimerization planes of ureido pyrimidone were parallel to each other, resulting in shielding, and the peaks of NH moved to high fields, moving to 13.05ppm, 11.72ppm and 9.74ppm respectively. . After adding 1 equivalent of benzo-18-crown 6 to the above solution, the peak of NH moved to 13.07ppm, 11.86ppm and 10.17ppm again in the downfield, indicating that after the potassium ion was captured by benzo-18-crown 6, the dimer Supramolecular polyme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com