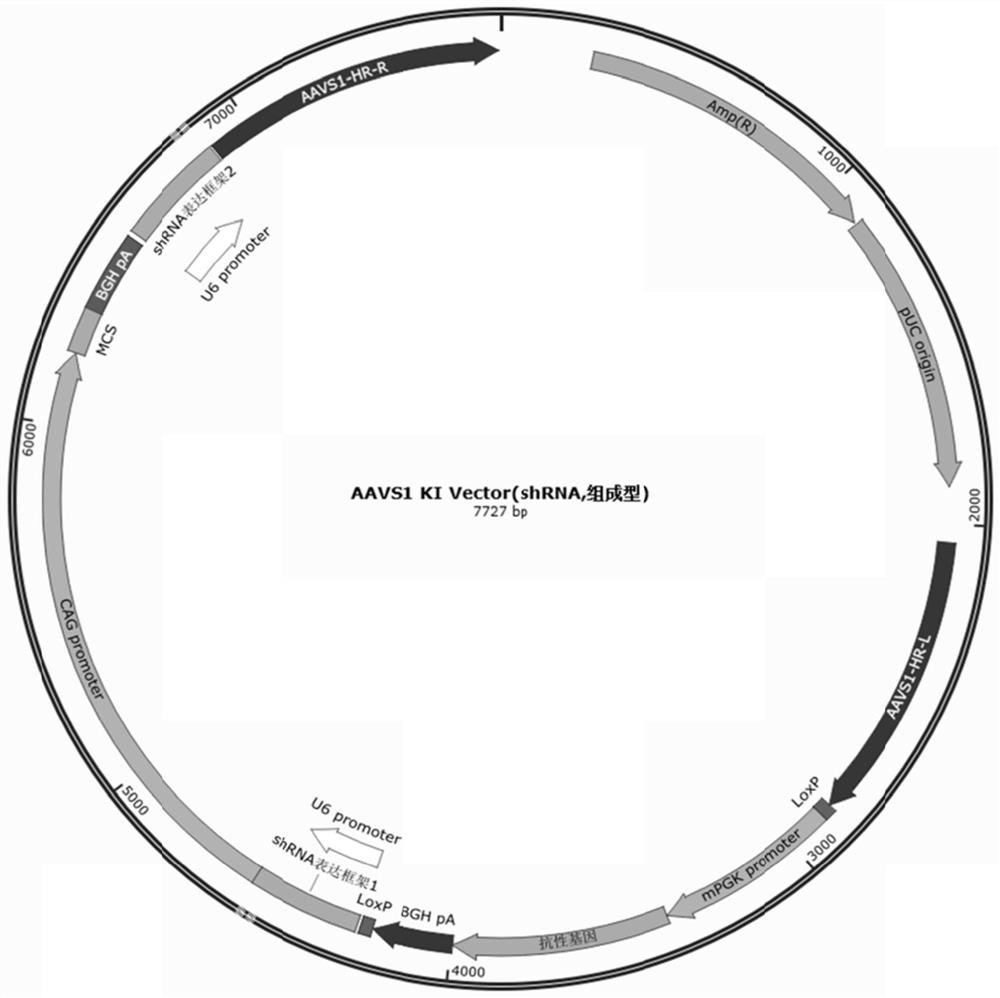
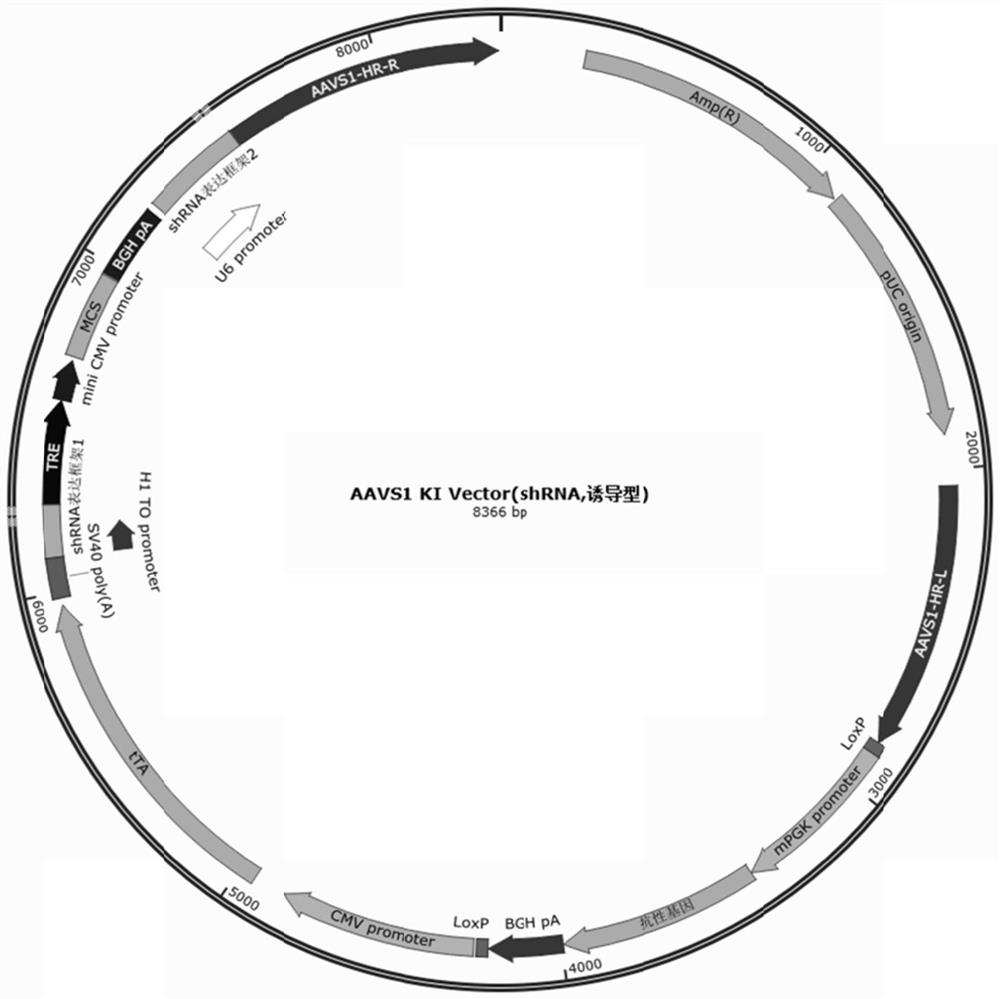
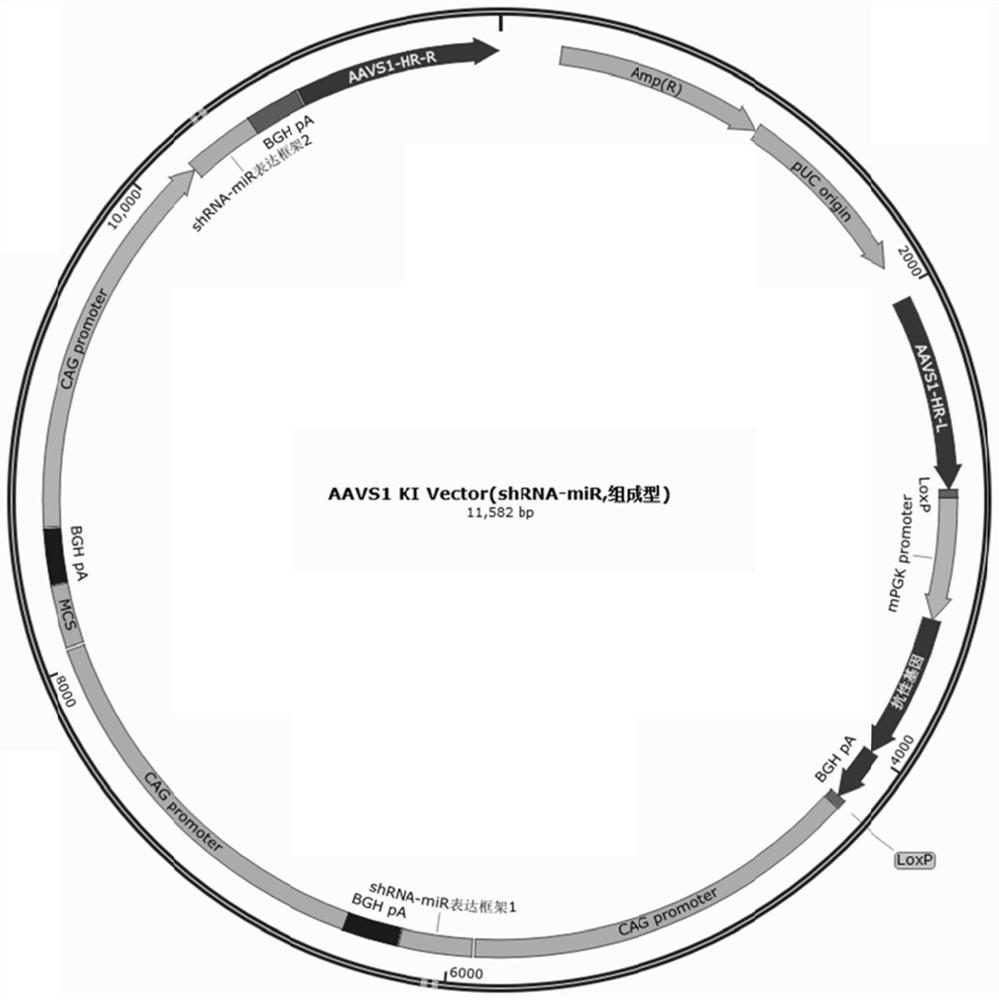
Pluripotent stem cells expressing shRNA/shRNA-miR targeting CTLA-4 or derivatives thereof
A CTLA-4, pluripotent stem cell technology, applied in DNA/RNA fragments, cells modified by introducing foreign genetic material, recombinant DNA technology, etc., can solve tumorigenicity and virus infection, loss of antigen presentation ability, Insufficient immune compatibility, etc., to relieve immune suppression, restore T cell activity, and remove tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0299] Example 1 Detection method for expressing pluripotent stem cells comprising CTLA-4 repressor molecule exosomes or derivatives thereof
[0300] Knock the protocols of each experimental group in Table 6 and Table 7 into the genomic safety site AAVS1 of iPSCs, MSCs, NSCs, and EBs cells, culture in a 37°C, 0.5% CO2 incubator, collect the culture supernatant, and use the exosome extraction kit (BestBio, lot#BB-3901) extract exosomes in the culture supernatant after culturing pluripotent stem cells expressing exosome-encapsulated CTLA-4 repressor molecules and their derivatives. The culture supernatant was centrifuged at 3000g for 15min at 4°C. After collecting the supernatant, centrifuge at 10,000g for 20min at 4°C. After collecting the supernatant, add extract A at a ratio of 4:1, invert upside down for 1min, and overnight at 4°C. Centrifuge at 10,000 g for 60 min at 4°C to collect the exosome pellet.
[0301] The effect of blocking CTLA-4 repressor molecules expressed by ...
Embodiment 2
[0306] Example 2 Anti-tumor effect of pluripotent stem cells expressing CTLA-4 repressor molecules or derivatives thereof
[0307] Knock the protocols of each experimental group in Table 6-Table 7 into the genomic safety site AAVS1 of iPSCs, MSCs, NSCs, and EBs cells to obtain cells expressing CTLA-4 repressor molecules, using 51 Cr release test was used to test its antitumor effect.
[0308] The N (control) group refers to T cells not treated with the culture supernatant of pluripotent stem cells expressing CTLA-4 repressor molecules.
[0309] Table 9 Effects of CTLA-4 repressor molecules expressed by each experimental group on T cells killing tumor cells
[0310]
[0311]
[0312] Through the above experiments, it can be proved that the exosome-encapsulated CTLA-4 expressed by the pluripotent stem cells prepared in the present invention or their derivatives can effectively inhibit CTLA-4 and play an anti-tumor effect.
Embodiment 3
[0313] Example 3 CTLA-4 repressor molecules are applied in the treatment of various tumors
[0314] We selected B2M and CIITA gene knockout protocol groups (Aa3, Ab3, Ac3, Ad3) in immune-compatible cells (hPSCs, MSCs, NSCs, EBs) for testing.
[0315] In the humanized NSG mouse tumor model, we injected each group of experimental cells to observe its therapeutic effect on RCC kidney cancer, MC colon cancer and HCC liver cancer. In order to avoid immune compatibility problems, the immune cells, hPSCs and hPSCs-derived derivatives we use are all derived from the same person.
[0316] The N (control) group refers to the NSG mouse tumor model that was not injected with the experimental cells. Independent samples T-test (*p<0.01).
[0317] Table 10 Anti-tumor effect of pluripotent stem cells expressing CTLA-4 repressor molecules or derivatives thereof
[0318]
[0319] Through the above experiments, it can be proved that the exosome-encapsulated CTLA-4 expressed by the pluripot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com