Photoactive nano composite as well as preparation method thereof and application thereof
A nanocomposite and photoactive technology, which is applied in the fields of polymer chemistry and biomedical engineering, can solve the problems of complex preparation, poor water solubility, and low stability, and achieve good biocompatibility, simple process, and wide application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
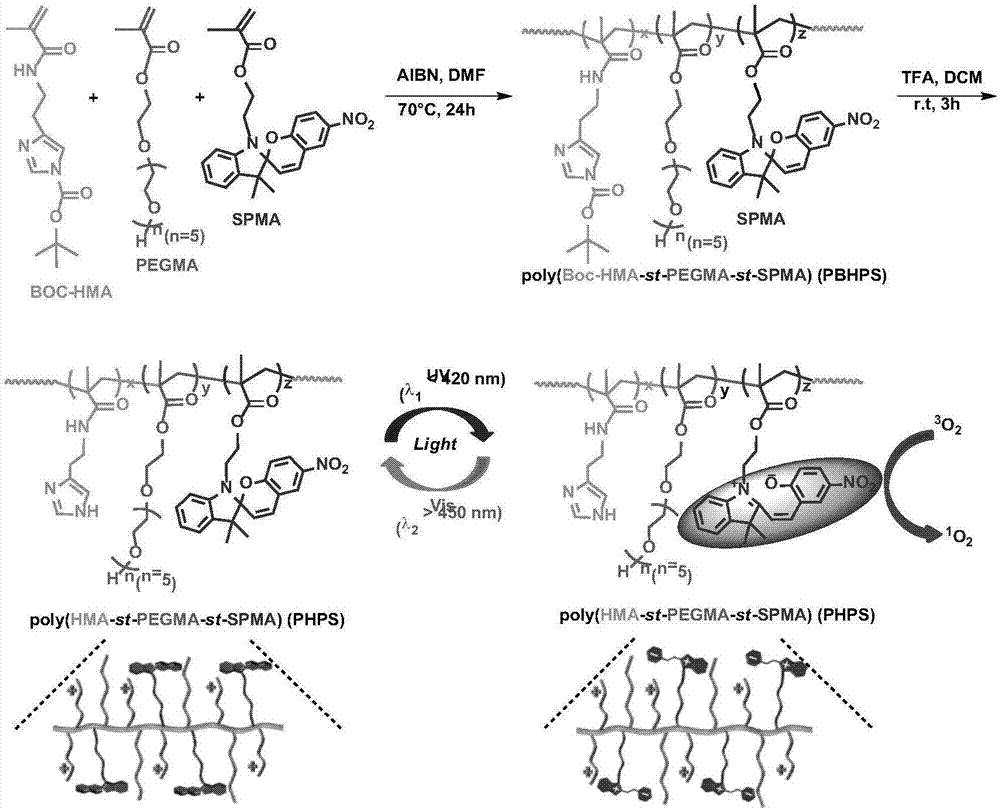
[0060] Step 1: Preparation of the spiropyran monomer constituting the photosensitive segment of the cationic polymer
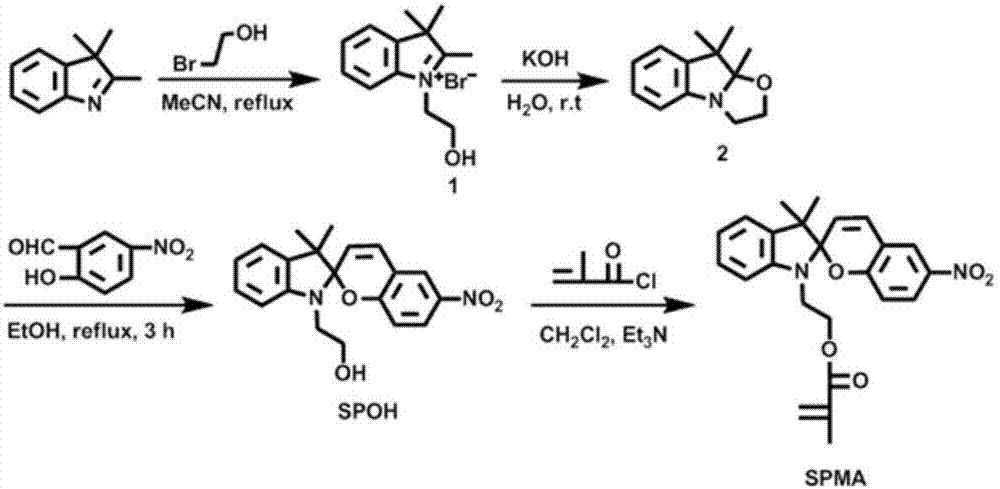
[0061] That is, 1'-(2-methacryloyloxyethyl)-3',3'-dimethyl-6-nitro-spiro(2H-1-benzopyran-2',2'-indole Phyloline) (SPMA) monomer and preparation thereof, one of the monomers that participate in polymerization to form cationic polymer is SPMA among the present invention, and its structural formula is as figure 1 Shown, the preparation method of SPMA comprises the steps:
[0062] (1) Synthesis of 1-(2-hydroxyethyl)-2,3,3-trimethyl-3H-indolium bromide:
[0063] Dissolve 3.18g of 2,3,3,-trimethyl-3H-indole and 3.125g of 2-bromoethanol in 30mL of acetonitrile and put them in a 100mL three-necked flask with a condenser tube, pass N 2 Protection, slowly heated to 80 ° C, the reaction 24h. After the reaction, the reaction system was slowly cooled to room temperature, and the acetonitrile solvent was removed by rotary evaporation to obtain a dark red solid. Add 50 m...
Embodiment 2
[0141] Both step 1 and step 2 of this embodiment are the same as that of embodiment 1.
[0142] Step 3 is the same as that of Example 1, except that: (1) In the synthesis of the polymer (PBHPS) protected by Boc group, raw materials Boc-HMA (33mg, 0.118mmol), PEGMA (53.2mg, 0.145 mmol), SPMA (12.4 mg, 0.030 mmol), resulting in a brown solid (54 mg, 55.3%).
[0143] Performance test: pass 1 H NMR (400M Hz, CDCl 3 ) to characterize its structure, such as Figure 13 As shown, the result shows that the structure is correct, and at the same time, the proportion of each component is calculated according to the characteristic peak displacement integral area of each group. The molecular weight of polymer PBHPS by size exclusion chromatography (GPC) (M n , M w ) and molecular weight distribution index (PDI) were tested.
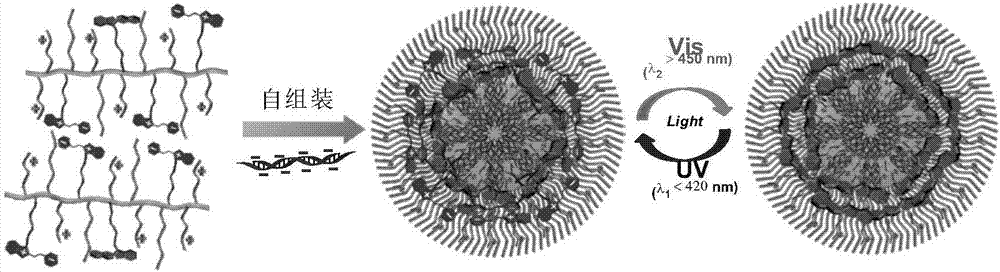
[0144] (2) Synthesis of Cationic Polymer (PHPS)
[0145] 40 mg of Boc group-protected copolymer PBHPS was dissolved in 0.5 mL of dichloromethane while cooling...
Embodiment 3
[0151] Both step 1 and step 2 of this embodiment are the same as that of embodiment 1.
[0152] Step 3 is the same as that of Example 1, except that: (1) in the synthesis of the polymer (PBHPS) protected by Boc group, raw materials Boc-HMA (35mg, 0.126mmol), PEGMA (50.2mg, 0.139 mmol), SPMA (5.9 mg, 0.0139 mmol), resulting in a brown solid (51 mg, 56%).
[0153] Performance test: pass 1 H NMR (400M Hz, CDCl 3 ) to characterize its structure, such as Figure 13 As shown, the result shows that the structure is correct, and at the same time, the proportion of each component is calculated according to the characteristic peak displacement integral area of each group. By size exclusion chromatography (GPC) to the molecular weight (M) of polymer PBHPS in the foregoing three embodiments n , M w ) and molecular weight distribution index (PDI) were tested. The ratio of each component and the GPC test results are shown in Table 2.
[0154] Table 2 Composition of each part of Boc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com