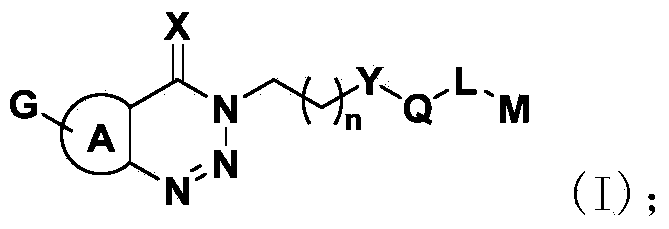
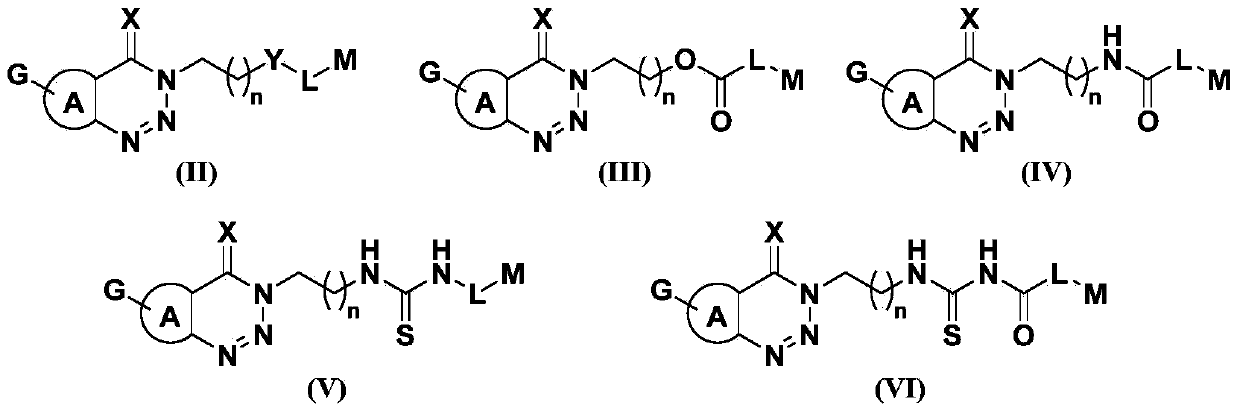
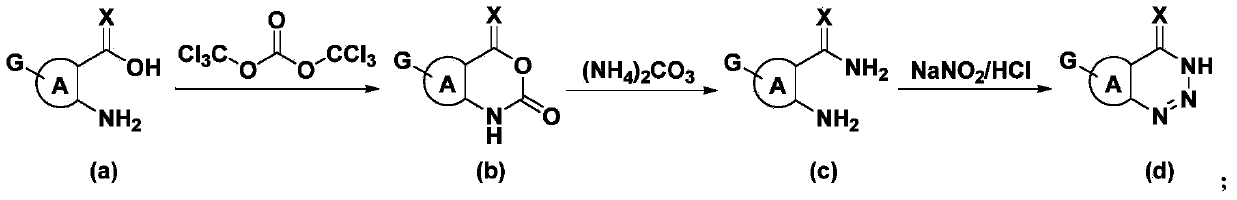
Triazine heterocyclic compound with nematicidal activity as well as preparation method and application of triazine heterocyclic compound
A compound and heterocyclic group technology, applied in the field of triazine heterocyclic compounds, can solve problems such as pollution, increased difficulty in nematode control, and low safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The preparation method of the compound of the present invention
[0112] The compound represented by the general formula (I) of the present invention can be prepared by the following method, but the conditions of the method, such as the amount of reactant, solvent, base, compound used, reaction temperature, reaction time required, etc. are not limited to the following explanations . The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0113] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent at a reaction temperature of -20-120°C (preferably -10-0°C or 20-30°C or 80-100°C). The reaction time is usually 2 to 24 hours, preferably 4 to 18 hours.
[0114] The base used in the reaction ...
Embodiment 1
[0155] Example 12-cyanoimino-3-(N-3-(4-oxo-7-chlorobenzo[d][1,2,3]triazin-3(4H)-yl)propyl)) -1,3-Thiazolidin-4-one
[0156] 1.1 Preparation of 7-chlorobenzo[d][1,2,3]triazin-4(3H)-one
[0157] 1.1.1 Preparation of 7-chloroisatoic anhydride
[0158]
[0159] Add 6.864g (40mmol) of 2-amino-4-chlorobenzoic acid into 100ml of tetrahydrofuran, stir it to form a suspension, lower it to -10°C, control the temperature at -10~-5°C, and slowly add 11.88g (40mmol ) BTC in 20ml tetrahydrofuran solution, after the addition was completed, it was placed at room temperature for reaction, and TLC followed the reaction process. After the reaction, the solvent was evaporated under reduced pressure, 150ml of anhydrous diethyl ether was added to the residual solid, stirred thoroughly, suction filtered, the filter cake was washed with anhydrous diethyl ether, and dried to obtain 6.718g of white solid, with a yield of 85%. 1 HNMR (400MHz, DMSO-d 6 )δ11.84(s,1H),7.92(d,J=8.4Hz,1H),7.30(dd,J 1...
Embodiment 2
[0175] Example 22-cyanoimino-3-(N-(4-(4-oxo-7-fluorobenzo[d][1,2,3]triazin-3(4H)-yl)butyl) )-1,3-thiazolidin-4-one
[0176]
[0177] The synthesis of the target compound is similar to step 1.4 of Example 1, except that 3-(4-bromobutyl)-7-fluorobenzo[d][1,2,3]triazine-4(3H) -one instead of 3-(3-bromopropyl)-7-chlorobenzo[d][1,2,3]triazin-4(3H)-one. Yellow solid, yield 53%. 1 HNMR (400MHz, DMSO-d 6 )δ8.33(dd,J 1 =8.8Hz,J 2 =5.6Hz,1H),8.07(dd,J 1 =8.8Hz,J 2 =2.4Hz,1H),7.81(td,J 1 =8.8Hz,J 2 =2.4Hz, 1H), 4.39(t, J=6.8Hz, 2H), 4.31(s, 2H), 3.65(t, J=6.8Hz, 2H), 1.90-1.75(m, 2H), 1.69-1.56 (m,2H); 19 FNMR (376MHz, DMSO-d 6 )δ-101.9(td,J 1 =8.6Hz,J 2 =5.6Hz).HRMS(ES-)C 15 h 12 N 6 o 2 FS(M-H) – , calculated value: 359.0726, measured value: 359.0723.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com