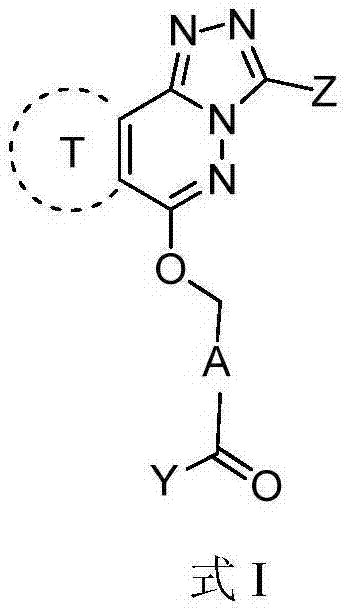
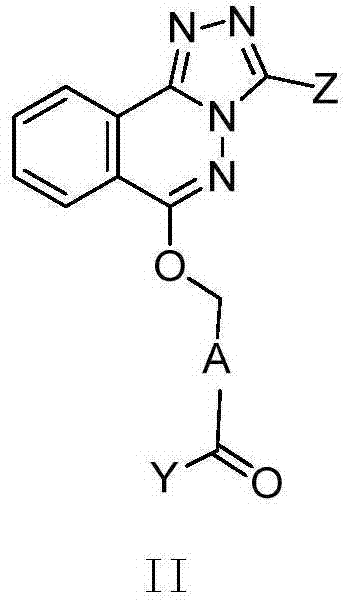
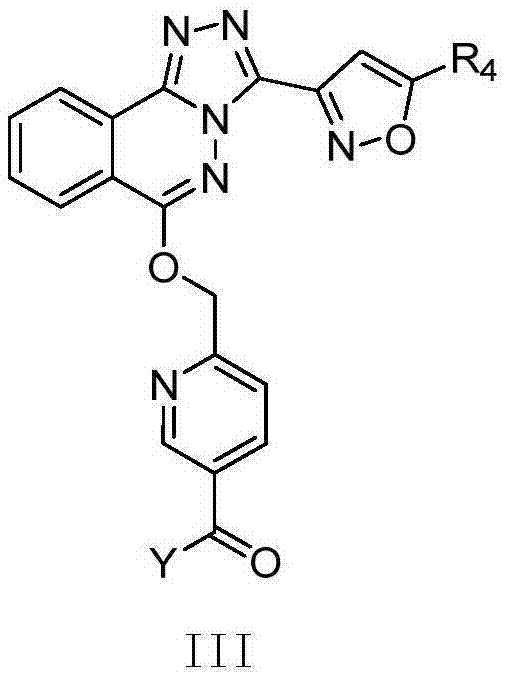
Phthalazine derivatives, and preparation method, pharmaceutical composition and application thereof
A technology of compounds and hydrates, applied in the field of thallazine derivatives, can solve problems such as animal fear and anxiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0310] 6-((3-(5-Methylisoxazol-3-yl)-[1,2,4]triazol[3,4-a]phthalazine-6-oxy)methylene)-N - Morpholine Niacinamide (01)
[0311] Compound A4 (100 mg, 0.248 mmol), HOBt (68 mg, 0.496 mmol) and EDCI (95 mg, 0.496 mmol) were sequentially added to 5 mL of DMF, and stirred at room temperature for 10 minutes under argon protection. 4-Aminomorpholine (CAS: 4319-49-7) (30.6 mg, 0.3 mmol) and N,N-diisopropylethylamine (130 mg, 0.992 mmol) were sequentially added to the mixture, followed by stirring at room temperature for 12 hours. TLC (developing solvent: dichloromethane:methanol=10:1, Rf=0.4) showed that the starting material was completely reacted. Add 25 mL of dichloromethane to the reaction solution, then pour the reaction solution into 30 mL of water, and adjust the pH to 5-6 with 2N citric acid. The organic layer was separated and washed with water (20 mL×2). The organic layer was dried over anhydrous sodium sulfate and concentrated. The residue was purified by a preparative pl...
Embodiment 2
[0313] (R)-N-(1-hydroxy n-propanol-2-yl)-6-((3-(5-methylisoxazol-3-yl)-[1,2,4]triazole[ 3,4-a]phthalazine-6-oxo)methylene)nicotinamide (02)
[0314] The experimental operation was as described in Example 1: the raw materials were compound A4 and D-aminopropanol (CAS: 35320-23-1) to obtain product (02) (35 mg, 20.5%), which appeared as a white solid. 1 H NMR(400MHz,DMSO-d6)δ:9.06~9.05(d,1H),8.60~8.58(d,1H),8.37~8.35(d,2H),8.27(d,1H),8.13(t,1H ),8.00(t,1H),7.87~7.85(d,1H),6.93(s,1H),5.77(s,2H),4.77~4.74(t,1H),4.05~4.02(m,1H), 3.45(m,1H),3.37(m,1H),3.33(s,1H),2.58(s,3H),2.01~1.99(m,1H),1.15~1.13(d,3H); LC-MS: m / z(ES+)for C 23 h 21 N 7 o 4 460.14[M+1] + .
Embodiment 3
[0316] N-((1S,2S)-2-hydroxycyclopentyl)-6-((3-(5-methylisoxazol-3-yl)-[1,2,4]triazole[3, 4-a]phthalazine-6-oxo)methylene)nicotinamide (03)
[0317] The experimental operation was as described in Example 1: the raw materials were compound A4 and trans-(1S,2S)-2-amino-cyclopentanol hydrochloride (CAS: 68327-04-8) to obtain the product (75mg, 65.8%) Appearance is white solid. 1 H NMR (400MHz, DMSO-d6) δ: 9.04(d,1H), 8.60~8.58(d,1H), 8.48~8.46(d,1H), 8.38~8.36(d,1H), 8.28~8.25(d ,1H),8.15~8.12(t,1H),8.02~8.00(t,1H),7.87~7.85(d,1H),6.93(s,1H),5.78(s,2H),4.81~4.80(d ,1H), 4.02~3.99(m,2H), 2.58(s,3H), 2.02~1.99(m,2H), 1.87~1.46(m,4H); LC-MS: m / z(ES+) for C 25 h 23 N 7 o 4 486.19[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com