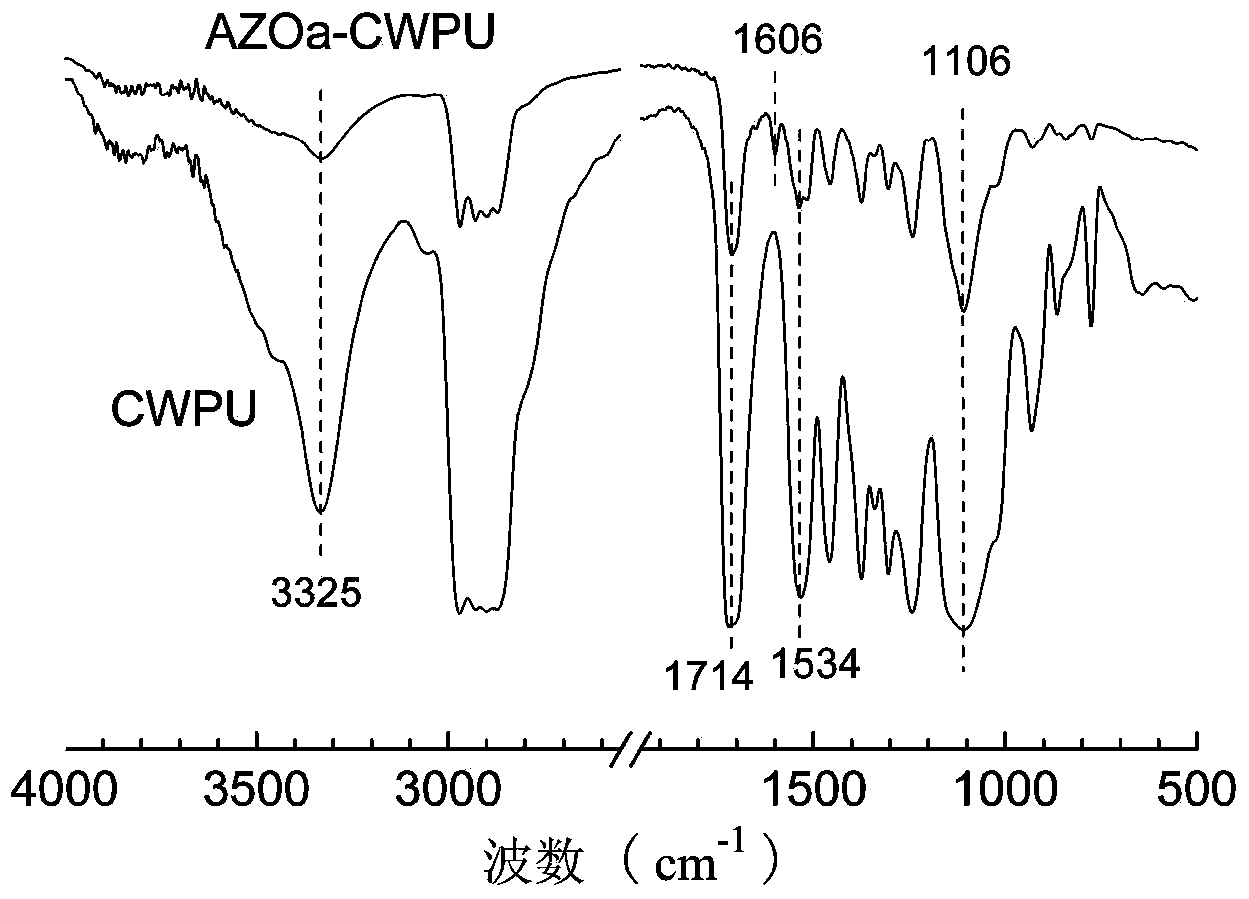
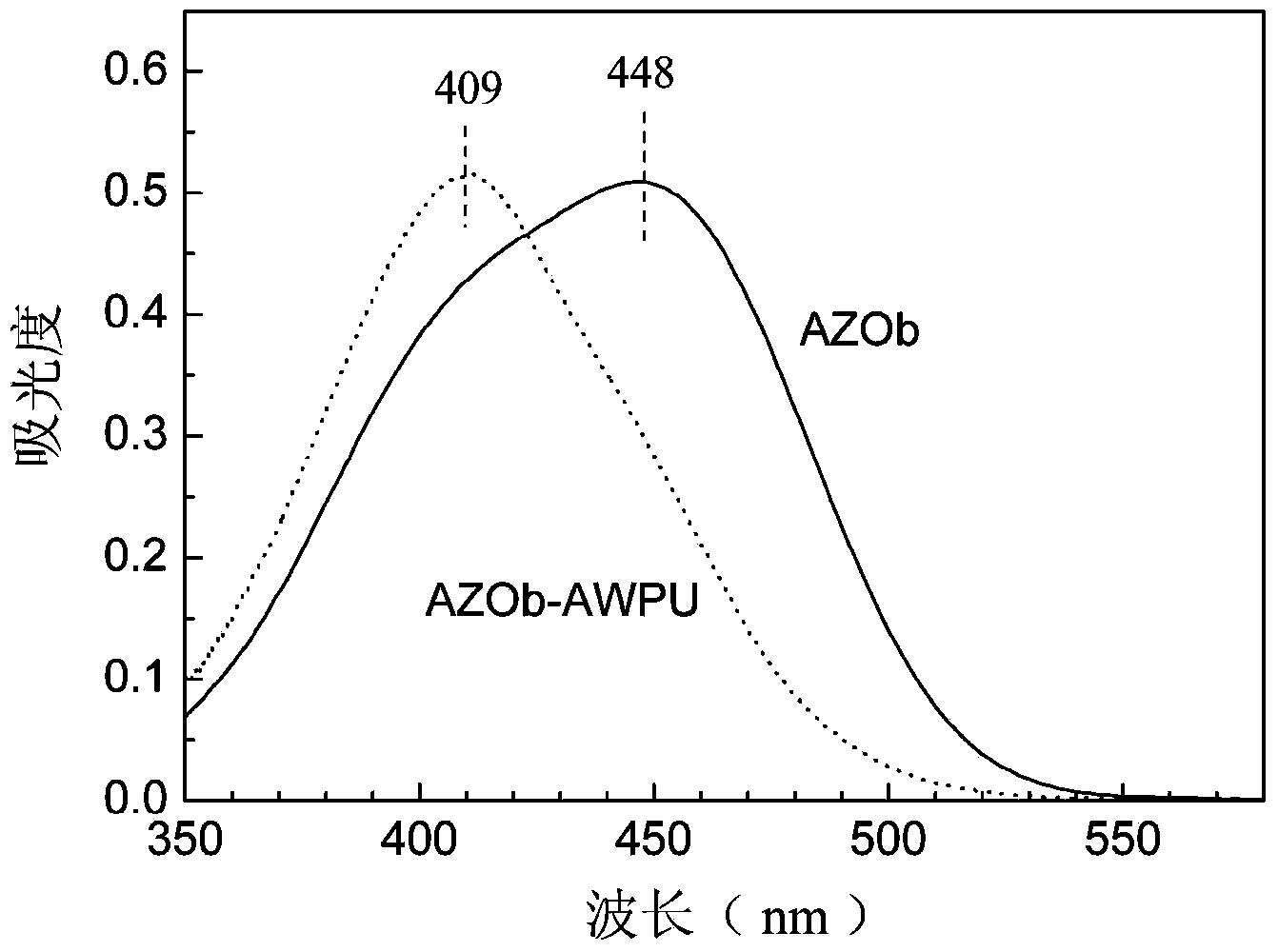
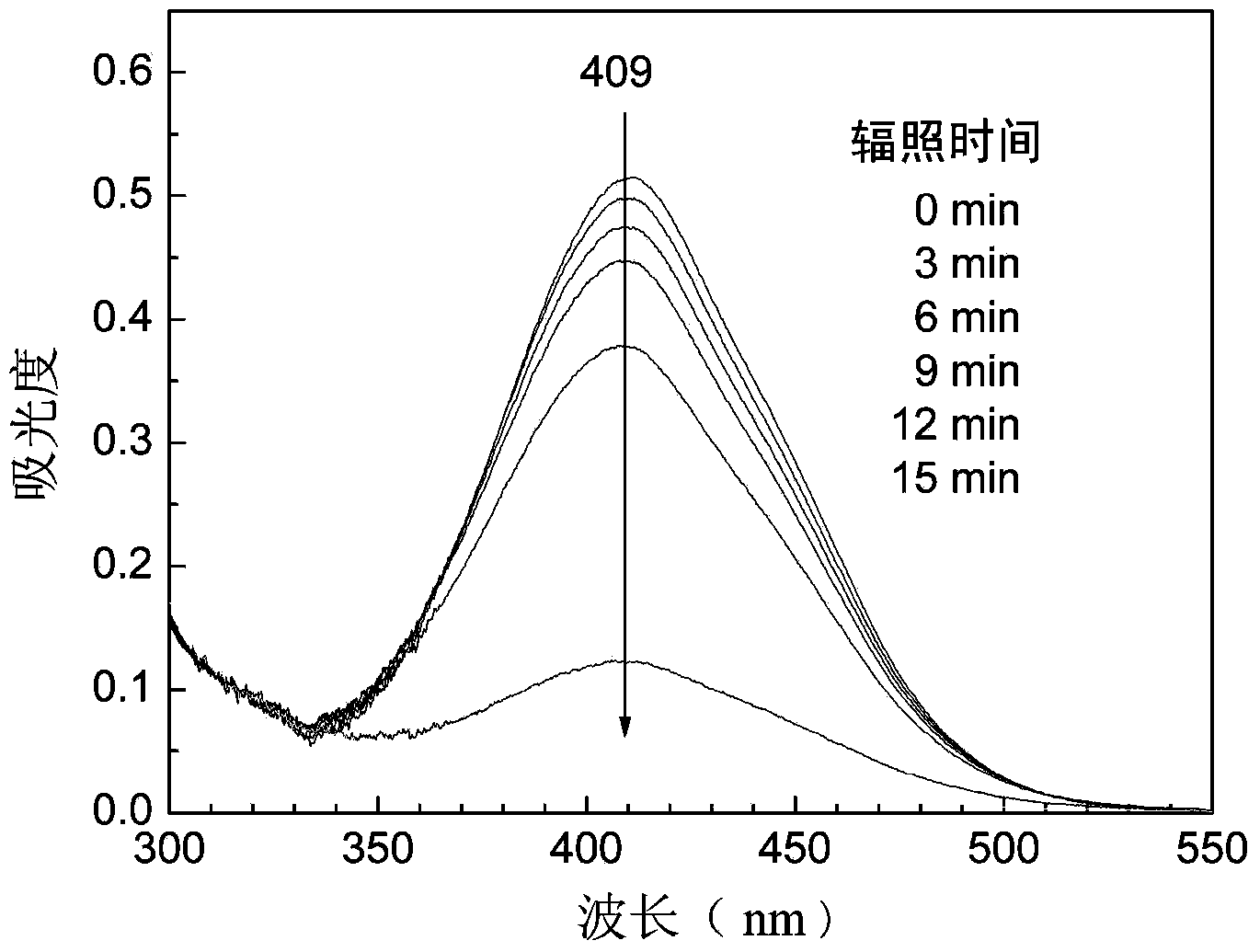
Preparation method for side-chain azo-type aqueous polyurethane
A water-based polyurethane and azo technology, which is applied in the field of side-chain azo-type water-based polyurethane and its preparation, can solve the problems of large amount of solvent used, low solid content of materials, water resistance of products, and problems with thermodynamic properties, and achieve uniform distribution , not easy to migrate, and the effect of maintaining the azo functional properties for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 15.35 grams of 4-ethylaniline into 100 mL of water, then slowly add 45 grams of concentrated sulfuric acid with a concentration of 98% by mass, stir magnetically for 5 minutes, cool down to 0-5°C, and then drop it within 1 minute from 8.75 A solution formed of 1 g of sodium nitrite and 15 mL of water was kept at 0-5°C for 1 hour to obtain a diazonium salt solution of 4-ethylaniline.
[0037] Add 5 g of acetic acid, 24 mL of methanol and 25 mL of water into 23.45 g of N,N-dihydroxyethylaniline, keep the reaction system at 0-5°C after forming a solution, and add the previously prepared 4-ethylaniline dropwise under magnetic stirring The diazonium salt solution, and the pH value of the system is adjusted to 6 by adding a substance concentration of 1M sodium hydroxide solution. Keep the reaction at 0-5°C for 2 hours, then filter, and recrystallize the obtained precipitate with a mixture of ethanol and water with a volume ratio of 2.5:1 to obtain azodiol N,N-dihydroxyeth...
Embodiment 2
[0042] Add 9 grams of aniline to 60 mL of water, then slowly add 29.4 grams of concentrated hydrochloric acid with a concentration of 37% by mass, dissolve and cool down to 0-5 °C. Under stirring, add a solution of 6.66 g of sodium nitrite and 10.5 mL of water dropwise within 1 minute, and keep at 0-5° C. for 1 hour to obtain a diazonium salt solution of aniline.
[0043] Add 30mL of methanol and 18mL of acetic acid into 15mL of water, then add 17.4g of N,N-dihydroxyethylaniline under stirring, cool down to 0-5°C after forming a solution, and add dropwise the diazonium salt solution of aniline prepared above , and the pH value of the system is adjusted to 6 by adding a substance whose concentration is 1M sodium hydroxide solution. Keep the system at 0-5°C for 2 hours, filter, wash the precipitate with water once, and then recrystallize the precipitate with a mixture of ethanol and water at a volume ratio of 2.5:1 to obtain azodiol N,N-dihydroxy Ethyl-4-phenylazoaniline (AZOb)...
Embodiment 3
[0050] Add 1.5g of butyl p-aminobenzoate and 3mL of acetic acid into 7.5mL of water, then slowly add 1.27g of concentrated hydrochloric acid with a concentration of 37% by mass, and cool down to 0-5°C after forming a solution; within 1 minute under stirring After the solution prepared by 0.53 g of sodium nitrite and 1.1 g of water was added dropwise, the reaction was maintained at 0-5° C. for 1 hour to obtain a diazonium salt solution of butyl p-aminobenzoate.
[0051] Add 1.44 g of N,N-dihydroxyethylaniline to 5 mL of methanol and 1 mL of acetic acid, stir to form a solution, then cool down to 0-5 °C, add the diazonium salt solution of butyl p-aminobenzoate prepared above dropwise , and the pH value of the system is adjusted to 6 by adding a substance whose concentration is 1M sodium hydroxide solution. Keep the system at 0-5°C for 2 hours, adjust the pH of the system to 7, filter the product, wash with water, and then recrystallize the product with ethanol to obtain azodiol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com