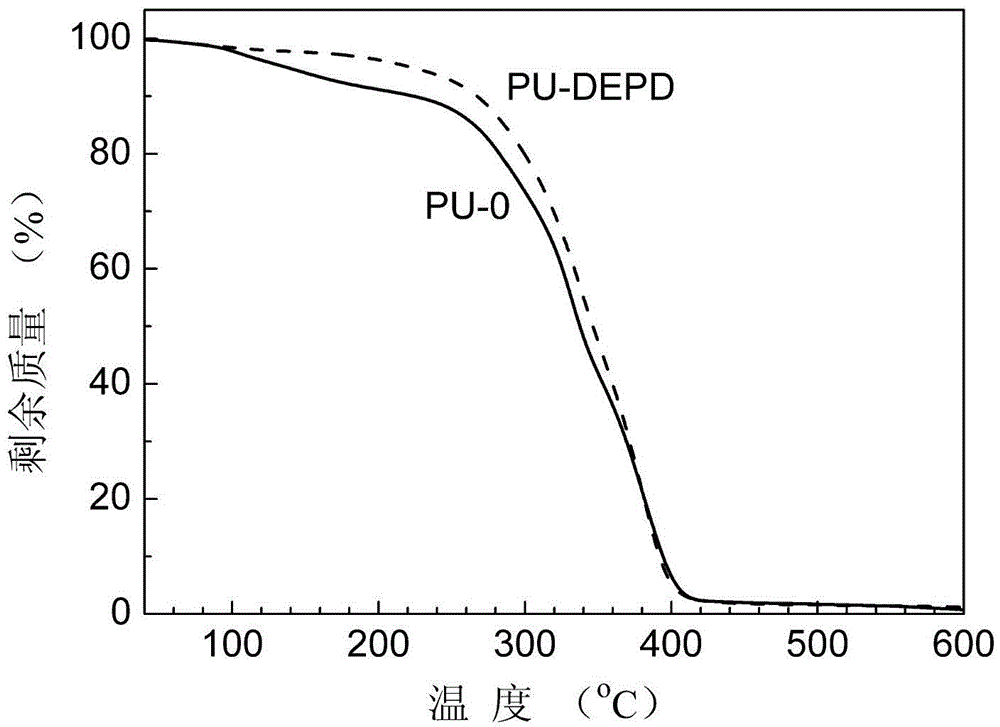
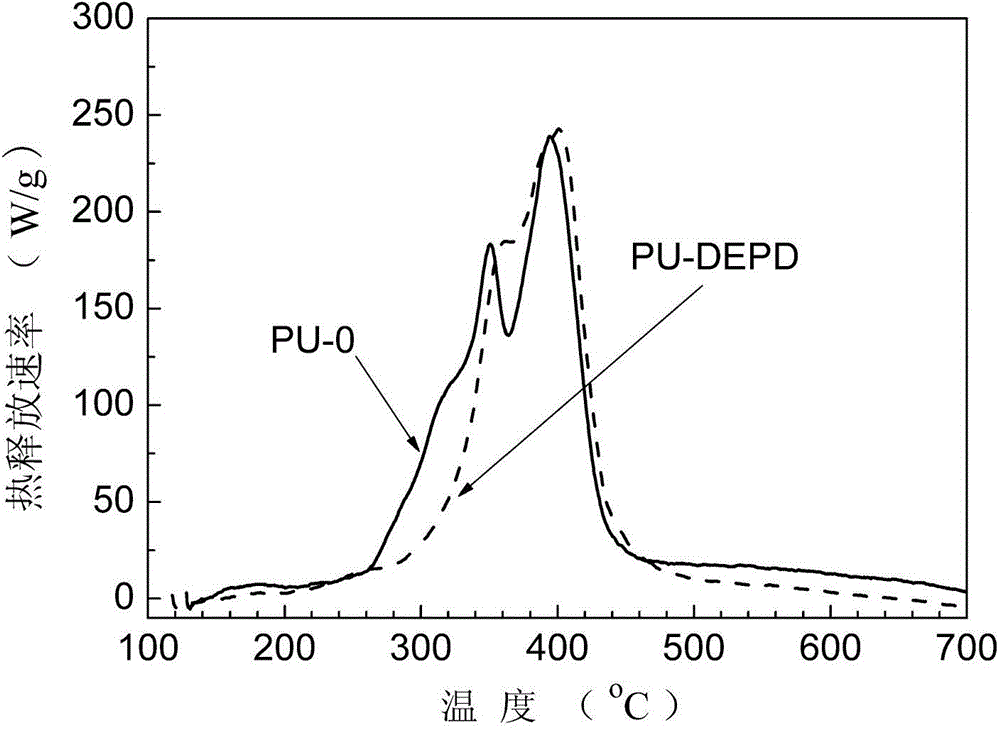
Flame-retardant glycol containing phenyl phosphate-based symmetrical structure and preparation method thereof
A technology of phenyl phosphate and symmetrical structure, which is applied in the field of flame retardant diols containing phenyl phosphate groups and its preparation, and can solve problems such as low reactivity, few types of flame retardant diols, and asymmetric hydroxyl structure. problems, to achieve the effect of easy-to-obtain raw materials, simple synthesis and post-treatment processes, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of flame retardant diol DEPD
[0027] Dissolve 12.41 grams of ethylene glycol in 50 mL of tetrahydrofuran, add 20.24 grams of triethylamine into a 500 mL three-necked flask, and place the reaction system in an ice-water bath; dissolve 21.98 grams of phenyl dichlorophosphate in 100 mL of tetrahydrofuran to form a solution , stirred and added dropwise the solution in the there-necked flask within 2 hours; the reaction system was raised to 25°C to continue the reaction for 16 hours; A reactive phosphorus-based flame-retardant diol product dihydroxyethyl phenyl phosphate DEPD with a symmetrical structure of phenyl phosphate groups was obtained with a yield of 92%.
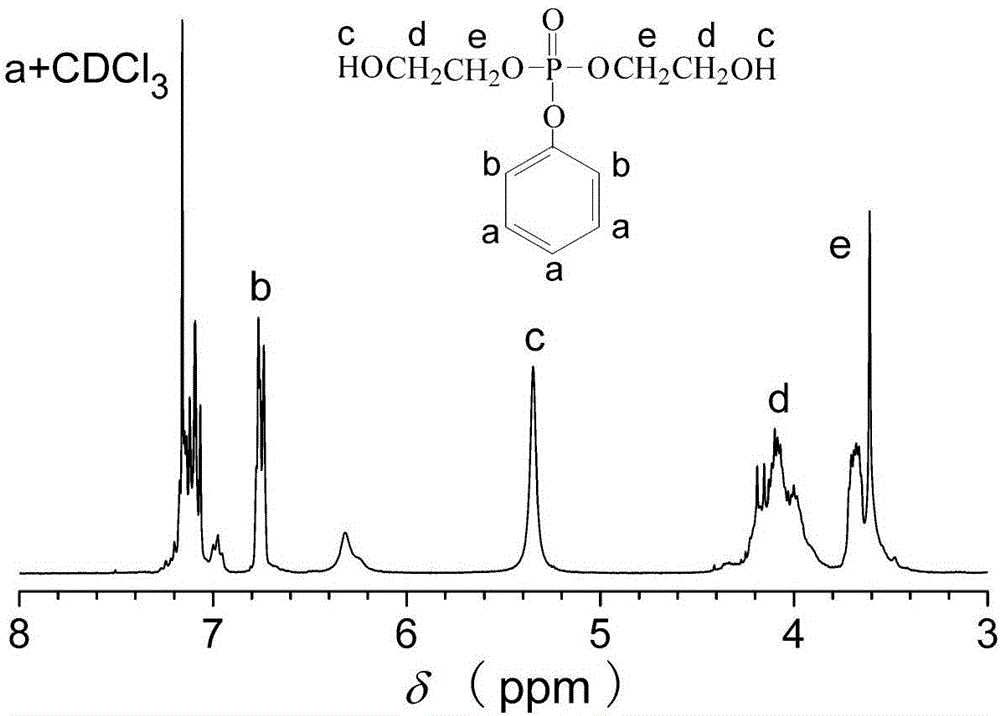
[0028] attached figure 1 The flame retardant diol DEPD and CDCl prepared in this embodiment are given 3 It is the proton nuclear magnetic resonance spectrogram of solvent, it can be known that the molecular structural formula of the product flame-retardant dihydric alcohol DEPD that m...
Embodiment 2
[0030] Example 2: Preparation of flame retardant diol DHPD
[0031] Dissolve 15 grams of 1,6-hexanediol in 50 mL of ethyl acetate, add 12.8 grams of triethylamine into a 500 mL three-neck flask, place the reaction system in an ice-water bath; dissolve 13.4 grams of phenyl dichlorophosphate Form a solution in 50mL of ethyl acetate, stir and drop the solution into the three-necked flask within 2 hours; raise the temperature of the reaction system to 25°C and continue the reaction for 16 hours; filter the filtrate to remove the precipitate and remove the solvent by vacuum distillation After ethyl acetate, dihydroxyhexylphenyl phosphate DHPD, a reactive phosphorus-based flame-retardant diol product with a symmetrical phenyl phosphate group structure, was obtained with a yield of 93%.
[0032] The molecular structural formula of the flame-retardant diol DHPD of the product of this example is confirmed by the hydrogen nuclear magnetic resonance spectrum:
[0033]
Embodiment 3
[0034] Embodiment 3: Preparation of flame retardant diol DDPD
[0035] Dissolve 18.03 grams of 1,4-butanediol in 50 mL of tetrahydrofuran, add 20.21 grams of triethylamine into a 500 mL three-necked flask, and place the reaction system in an ice-water bath; dissolve 21.98 grams of phenyl dichlorophosphate in 50 mL A solution was formed in tetrahydrofuran, stirred and added dropwise to the three-necked flask within 2 hours; the temperature of the reaction system was raised to 35°C to continue the reaction for 16 hours; The reaction-type phosphorus-based flame-retardant diol product dihydroxybutyl phenyl phosphate DDPD with a symmetrical structure of phenyl phosphate group was obtained, and the yield was 92%.
[0036] Proton nuclear magnetic resonance spectrogram confirms that the molecular structural formula of the product flame-retardant dihydric alcohol DDPD of this embodiment is:
[0037]
[0038] If other conditions remain unchanged, and the 1,4-butanediol used in it is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com