Nitrogen-containing heterocyclic compound with pesticidal activity, preparation and application thereof
A compound and six-membered heterocycle technology, applied in the field of new insecticides, can solve problems such as narrow insecticidal spectrum and limited drug selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
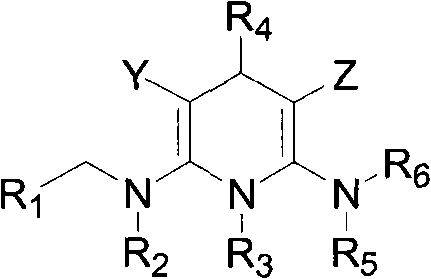
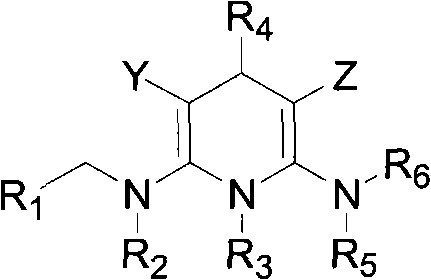
[0081] The preparation method of the compound of the present invention
[0082] The synthetic method of general formula (A) compound is as follows:
[0083]
Embodiment 1-22
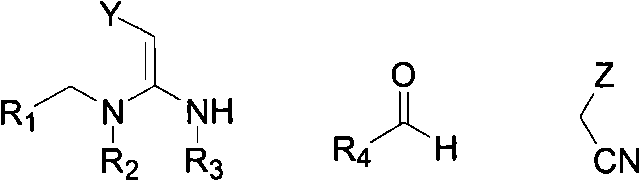
[0086] Example 1-22: Preparation of 1,4-dihydropyridine nitromethylene compounds
[0087]
[0088] Dissolve aromatic aldehyde (4.5mmol, 150mol%) in absolute ethanol (30mL) solution at room temperature, then add malononitrile (4.5mmol, 150mol%) to continue stirring, drop piperidine (10mol%) and stir After 2-4 hours, nitromethylene compound (3mmol, 150mol%) was added and the mixture was heated and refluxed for 3-24 hours. After cooling, it was filtered and washed 3 times with dichloromethane to obtain a solid compound.
[0089]
Embodiment 1
[0091] Example 1: A yellow solid was obtained after filtration with a yield of 82% and a melting point of 223.2-223.7°C. 1 H NMR (400MHz, DMSO-d 6 )δ3.93-4.01(m, 3H), 4.08-4.17(m, 1H), 4.72(s, 1H), 4.74(s, 2H), 6.54(s, 2H, NH 2 ), 6.98(dd, J=1.4, 7.8Hz, 1H), 7.15-7.23(m, 3H), 7.28(d, J=8.0Hz, 1H), 7.64(dd, J=2.6, 8.2Hz, 1H) , 8.28 (d, J=2.0Hz, 1H). 13 C NMR (100MHz, DMSO-d 6 )δ 41.52, 43.90, 51.05, 51.46, 60.12, 106.17, 121.21, 124.39, 126.69, 126.96, 128.73, 131.40, 139.48, 144.78, 149.51, 149.61, 149.90, 152.90. MS (ESI) m / z 409 (M+H). C 20 h 17 ClN 6 o 2 Calculated: C, 58.75; H, 4.19; N, 20.56. Found: C, 58.77; H, 3.89; N, 20.40.
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com