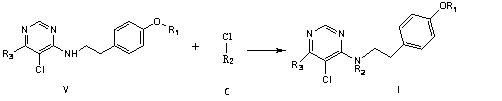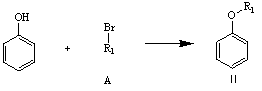Nicotine compound, preparation method and uses thereof
A technology for compounds and nicotines, applied in the field of nicotine compounds and their preparation, can solve the problems of reducing the killing rate of pests and the like, and achieve the effects of not easily producing drug resistance, reducing environmental pollution, and having high systemic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
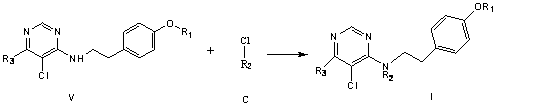
[0057] The preparation method of a nicotine compound of the present invention includes the following steps:
[0058] ① Dissolve 1mol phenol in 500-800ml N,N-dimethylformamide, add 1-5mol potassium carbonate, stir at 40-100℃ for 1-10h, then add 1mol compound A, at 50-100℃ React for 1-24 hours. After the reaction, the solvent is evaporated, water and dichloromethane are added for extraction, the solvent layer is taken, and the solvent is evaporated to obtain Intermediate II. The reaction formula is as follows:
[0059]
[0060] ② Dissolve 1 mol of Intermediate II in 500-800ml of 50% alcoholic water solution, add 1-1.05mol of paraformaldehyde and the same amount of sodium cyanide as paraformaldehyde, and react at 30-60℃ for 1-24h, After the reaction, dichloromethane is added for extraction, the solvent layer is taken, the solvent is evaporated, and the intermediate III is obtained by column chromatography. The reaction formula is as follows:
[0061] ;
[0062] ③ Dissolve 1 mol of comp...
Embodiment 1
[0093] The preparation steps of embodiment 1 nicotine compound (1) are as follows:
[0094] ① Dissolve 1mol of phenol in 500ml of N,N-dimethylformamide, add 1mol of potassium carbonate, stir at 40℃ for 10h, then add 1mol of trifluorobromomethane, react at 50℃ for 24 hours, distill out after the reaction is over Solvent, add 500ml of water and 500ml of dichloromethane for extraction, take the solvent layer and distill off the solvent to obtain 152.28g of Intermediate II;
[0095] ②Dissolve 152.28g of Intermediate II in 500ml of 50% methanol aqueous solution, add 1mol of paraformaldehyde and 1mol of sodium cyanide at 30℃, react for 24h, after the reaction is over, add 500ml of dichloromethane for extraction, take the solvent Layer, evaporate the solvent, 6:4 (volume ratio) ethyl acetate / petroleum ether column chromatography to obtain 190.65g intermediate III;
[0096] ③ Dissolve 1 mol of ethyl formylacetate in 300 ml of methanol, then add 1 mol of thiourea and 1 mol of sodium methoxid...
Embodiment 2
[0099] The preparation steps of embodiment 2 nicotine compound (8) are as follows:
[0100] ① Dissolve 1mol phenol in 500ml N,N-dimethylformamide, add 5mol potassium carbonate, stir at 100℃ for 1h, then add 1mol 1-trifluorobromoethane, and react at 100℃ for 1 hour. After the completion, the solvent was evaporated, 500ml of water and 500ml of dichloromethane were added for extraction, the solvent layer was taken, and the solvent was evaporated to obtain 170.72g of Intermediate II;
[0101] ②Dissolve 170.72g of Intermediate II in 800ml of 50% propanol aqueous solution, add 1.05mol of paraformaldehyde and 1.05mol of sodium cyanide at 60℃, react for 1h, after the reaction, add 500ml of dichloromethane for extraction , Take the solvent layer, evaporate the solvent, 6:4 (volume ratio) ethyl acetate / petroleum ether column chromatography to obtain 210.24g intermediate III;
[0102] ③ Dissolve 1 mol of ethyl acetoacetate in 2000 ml of methanol, then add 5 mol of thiourea and 5 mol of sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com