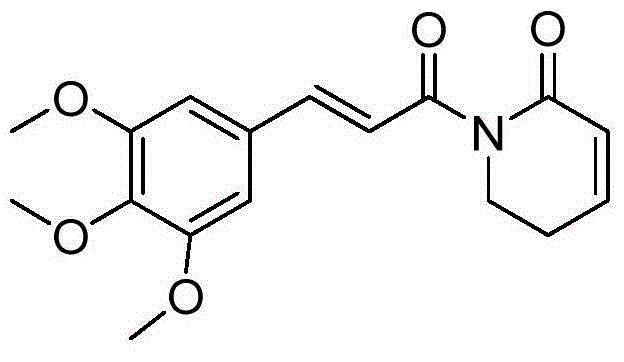
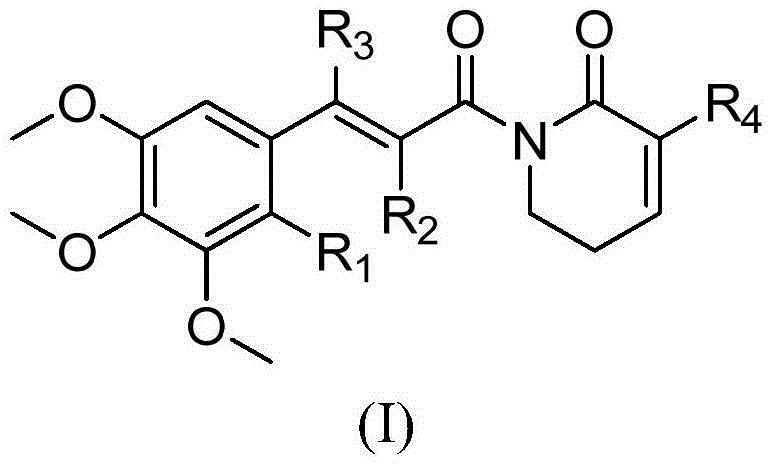
Piplartine analogue, and preparation method and application thereof
A technology of piperamide and analogs, which is applied to the application field of substituted piperamide compounds and their preparation, and the preparation of antitumor drugs, can solve the problem of enhancing the antitumor activity and selectivity of compounds, and the ineffectiveness of piperamide activities. higher question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
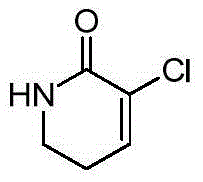
[0076]Example 1: Synthesis of 3-chloro-5,6-dihydro-2(1H)-pyridone
[0077]
[0078] Dissolve 25.0 g of 2-piperidone in 250 ml of chloroform, cool to 0°C, add 158.0 g of phosphorus pentachloride, stir for 15 minutes, then heat to reflux for 3 hours. After cooling, the reaction solution was slowly poured into 500 ml of ice water, the aqueous layer was extracted three times with 50 ml of dichloromethane, the combined organic phases were washed with saturated brine, dried, and the solvent was evaporated to obtain a yellow oily liquid.
[0079] Dissolve 4.0 g of the product from the previous step in 12 ml of DMF, add 3.6 g of anhydrous lithium carbonate, and react at 120 degrees for 7 hours. After cooling, it was poured into ice water, extracted three times with 50 ml of dichloromethane, washed with saturated brine, dried, concentrated, and the residue was purified by column chromatography to obtain 1.1 g of the product with a yield of 35.1%.
[0080] 1 H-NMR (CDCl 3 ):7.11-7...
Embodiment 2
[0081] Example 2: Preparation of 3-morpholino-5,6-dihydro-2(1H)-pyridone
[0082]
[0083] Dissolve 1.3 g of 3-chloro-5,6-dihydro-2(1H)-pyridone and 0.9 g of morpholine in 5 ml of DMF, add 1.5 g of anhydrous lithium carbonate, raise the temperature to 130°C, and react for 3 hours. After filtration, the solvent was evaporated under reduced pressure, and the residue was purified by column chromatography to obtain 0.6 g of white solid, with a yield of 30.2%.
[0084] 1 H-NMR (CDCl 3 ):6.15(s,1H),5.56(t,1H),3.82-3.85(m,4H),3.30-3.36(m,2H),2.87-2.90(m,4H),2.33-2.40(m,2H ).
Embodiment 3
[0085] Embodiment 3: Preparation of (E)-2-methyl-3-(3,4,5-trimethoxyphenyl)acrylic acid
[0086]
[0087] 2.0 grams of 3,4,5-trimethoxybenzaldehyde, 5.9 grams of 2-methylmalonic acid, 0.2 grams of piperidine and 20 milliliters of anhydrous pyridine were added to the flask, and refluxed for 24 hours. The solvent was evaporated under reduced pressure, the residue was dissolved in 50 ml of water, extracted three times with 20 ml of ethyl acetate, washed with water, dried, and the residue was purified by column chromatography to obtain 1.7 g of a white solid with a yield of 66.1%.
[0088] 1 H-NMR (CDCl 3 ):7.77(s,1H),6.69(s,2H),3.10(s,9H),2.19(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com