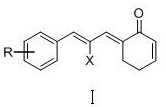
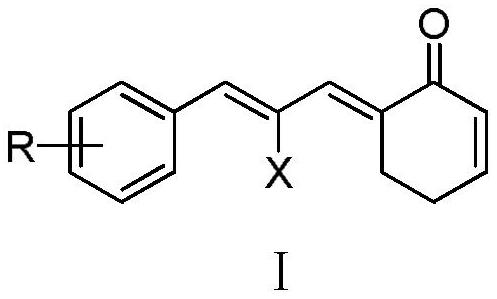
Phenylallylidene cyclohexenone derivatives and their preparation methods and uses
A technology of phenylallylidene cyclohexenone and base allylidene cyclohexenone is applied in the preparation of carbon-based compounds, the preparation of organic compounds, the preparation of carboxylic acid nitrile, etc. Limiting clinical applications, expensive metal catalysts, etc., to achieve the effect of inhibiting TrxR enzyme activity, inducing tumor cell apoptosis, and increasing ROS levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
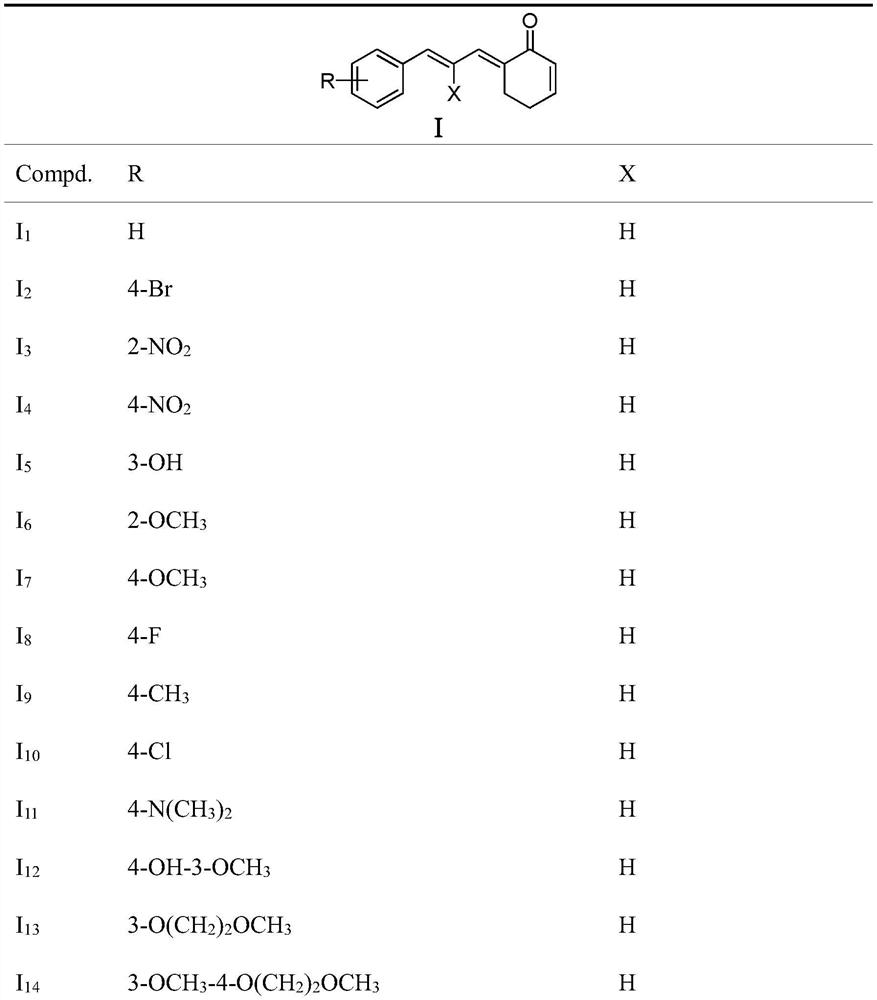
[0049] Embodiment 1 (E)-6-((E)-3-phenylallyl) cyclohex-2-enone (I 1 ) preparation
[0050]
[0051] Dissolve cyclohexen-2-one (0.48g, 5.0mmol) and triphenylphosphine (1.31g, 5.0mmol) in 50ml of anhydrous dichloromethane, and then add 5ml of 1mol / L TiCl at -50°C 4 After 15 minutes, use a constant pressure funnel to slowly add 30ml of cinnamaldehyde (1.32g, 10.0mmol) solution dissolved in dichloromethane dropwise. After half an hour, the reaction returns to room temperature, and the reaction continues for about 12h. TLC Monitor, after the reaction is complete, add an appropriate amount of 10% K 2 CO 3 After the solution continued to stir for about 5 minutes, the pH of the reaction solution was adjusted to 9, and then extracted with dichloromethane (50ml×2), and the extracted dichloromethane layer was washed with saturated brine (50mL), and the dichloromethane layer was collected. Dry over sodium sulfate, concentrate, and purify by column chromatography (EA:PE=1:3 as eluent...
Embodiment 2
[0089] Embodiment 2 MTT method is used to measure the inhibition rate of tumor cells and normal cell proliferation of the compounds of the present invention
[0090] The anti-proliferation activity of the compound of the present invention on 4 kinds of human cancer cell lines was evaluated by tetramethylazolidine blue colorimetric method (MTT) in vitro anti-tumor test. Perimine (PL) was used as the positive control drug. Human cancer cell lines: liver cancer cell SMMC7721, colon cancer cell HCT116, gastric cancer cell HGC-27, human cervical cancer cell Hela, normal human cells: human gastric mucosal epithelial cell GES-1.
[0091] The experimental method is as follows: take a bottle of cells in a good state in the exponential growth phase, add 0.25% trypsin to digest, make the adherent cells fall off, and make each ml contain 2×10 4 ~4×10 4 suspension of cells. The cell suspension was inoculated on a 96-well plate, 180 μL per well, and placed in constant temperature CO 2 Inc...
Embodiment 3
[0098] Example 3 Intracellular ROS Level Determination
[0099] ROS-Glo hydrogen peroxide assay (Promega, Southampton, UK) directly detects H in cells 2 o 2 Levels measure ROS changes. Cells were seeded into 96-well cell culture plates and incubated with test drugs (0.01-12.5 μM) for 24 hours. Hydrogen peroxide substrate solution was added to each well and kept at constant temperature CO 2 Incubate in an incubator at 37°C for 6 hours. After the incubation, add ROS-Glo detection solution to each well and incubate at room temperature for 20 minutes. Fluorescence was detected by a BioTek Synergy HT multimode microplate reader.
[0100] Select I in the compound of general formula I of the present invention 4 ~I 8 , I 11 ~I 15 , I 18 , I 19 As a representative, its ROS level in tumor cells was tested. DCFH-DA was used as a fluorescent probe to measure the changes of ROS in human cervical cancer Hela cells after drug treatment, and the changes of fluorescence intensity c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com