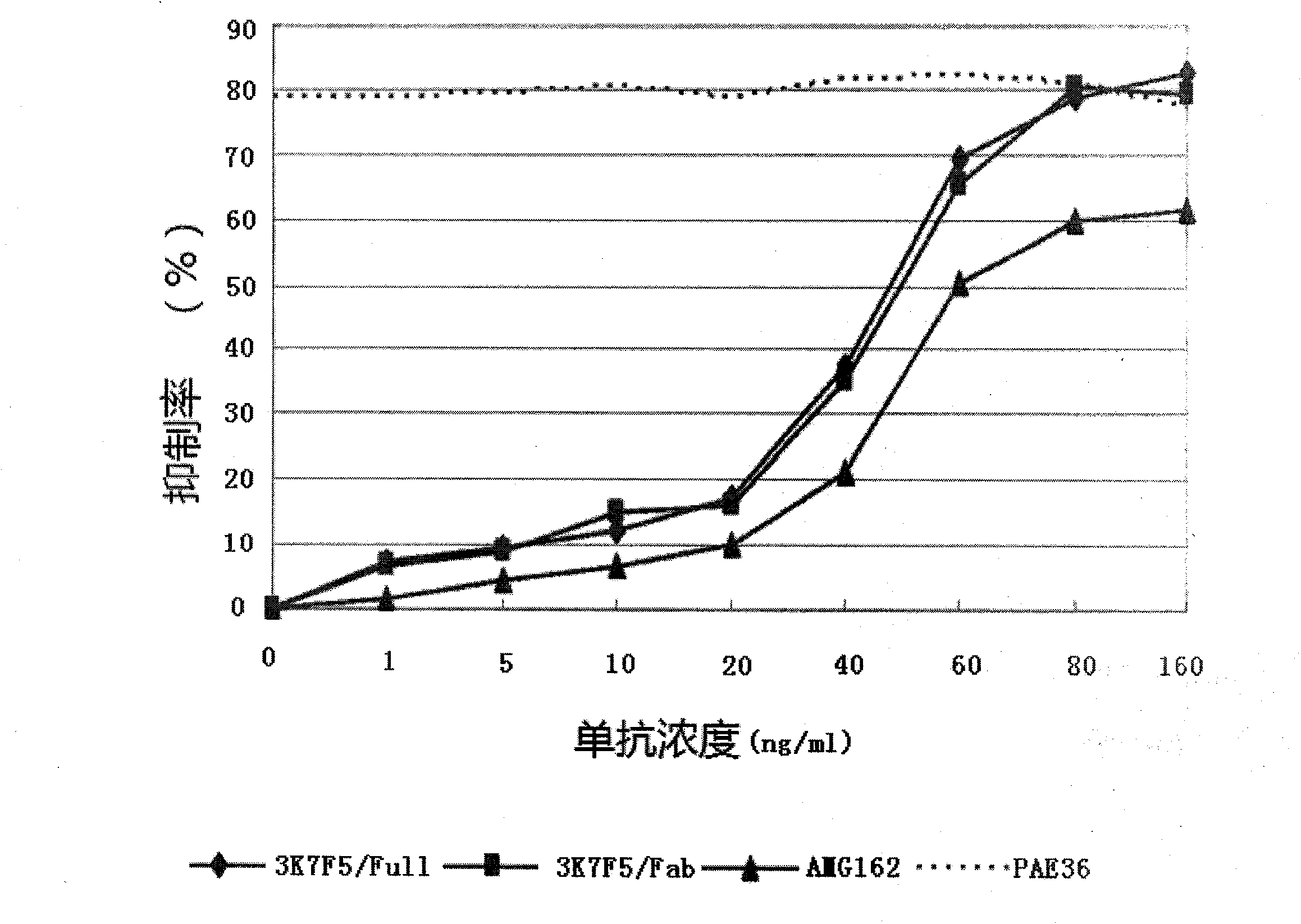
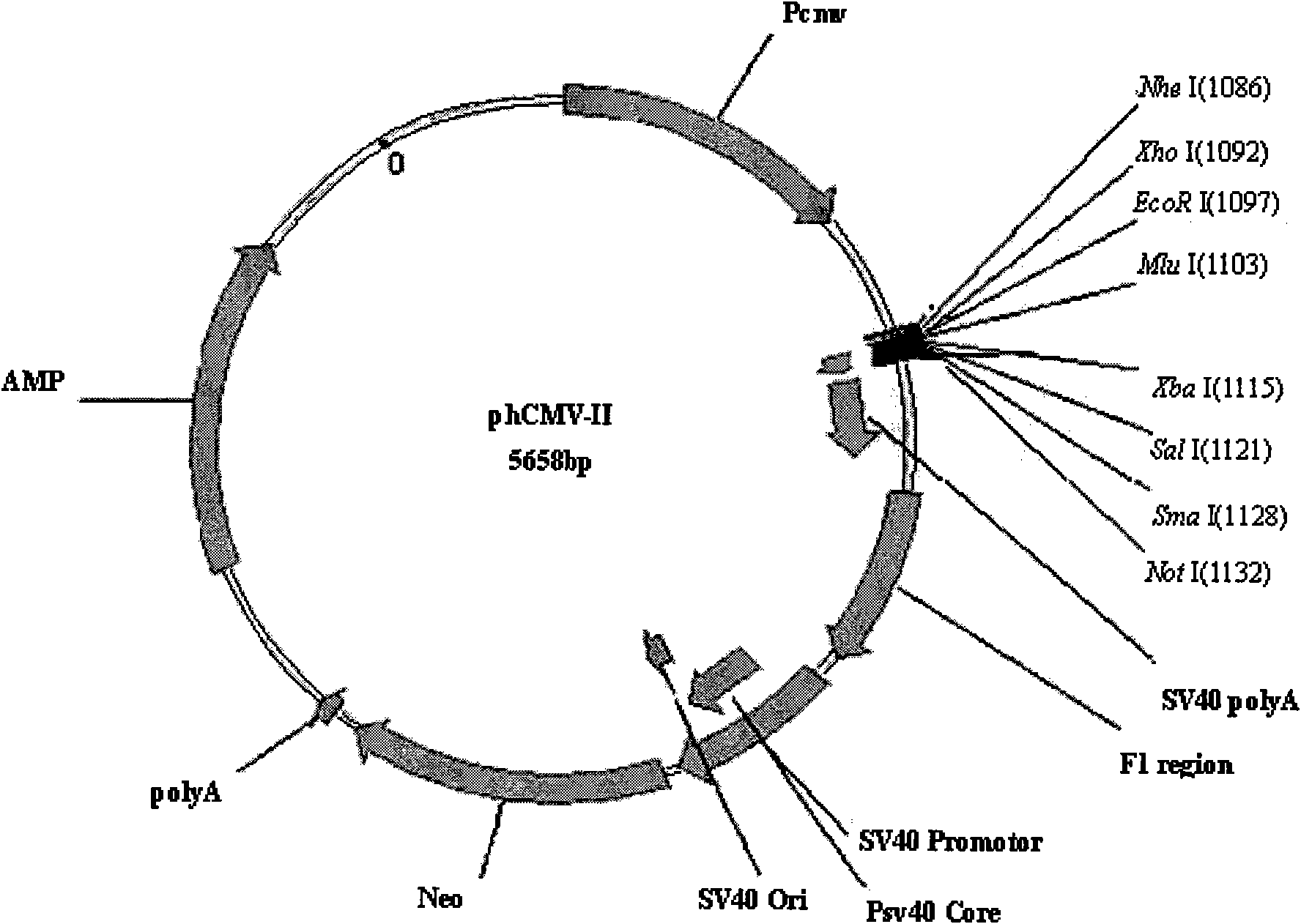
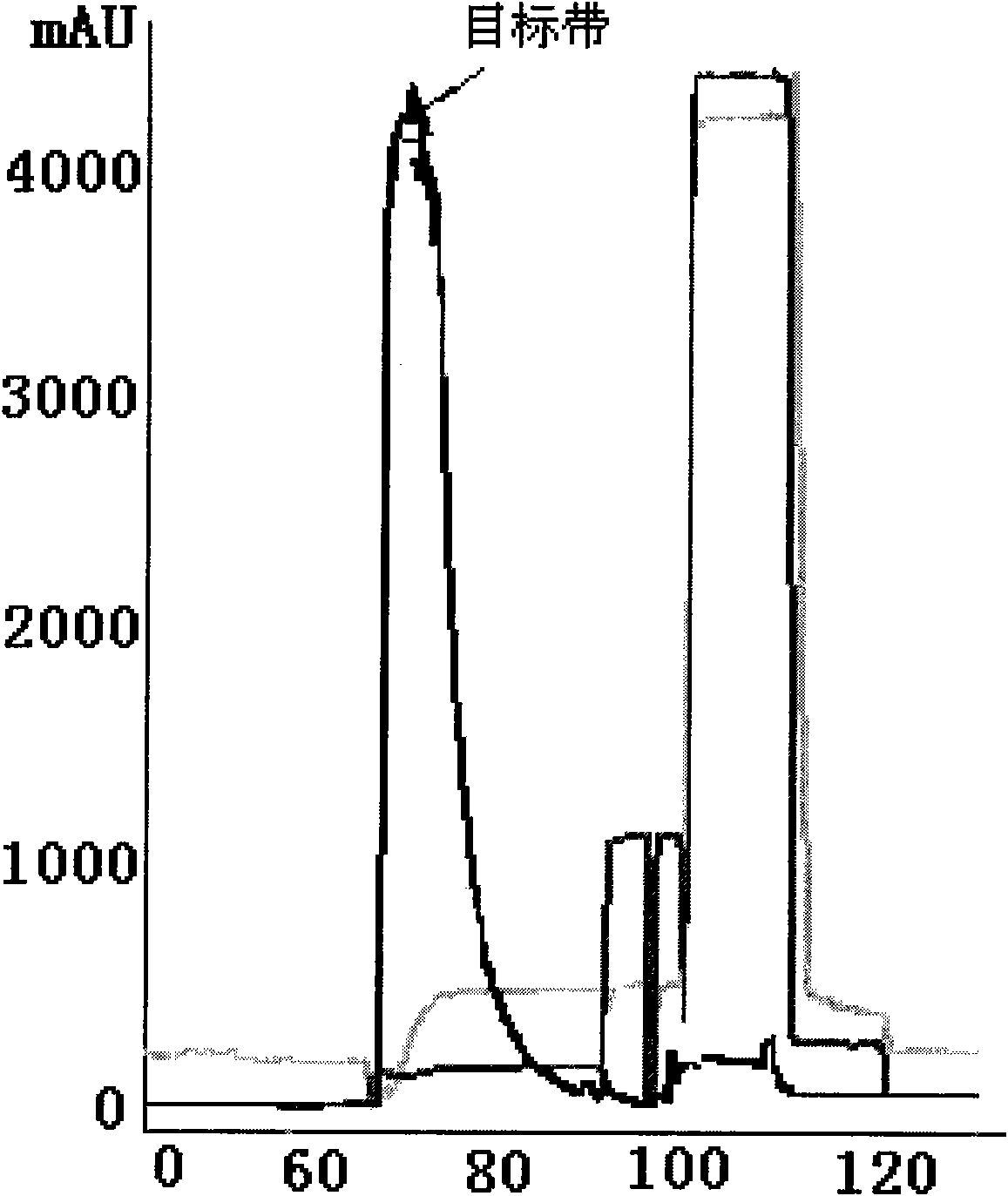
Anti human RANKL monoclonal antibodies developed by PAE technology and uses thereof
An antigen and antibody fragment technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody, resistance to vector-borne diseases, etc. Safety, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Panning for variable region fragments specific to human RANKL
[0081] (1) All-human source combination Construction of antibody library
[0082] Using more than 3,000 blood samples from blood donors from different provinces and different nationalities, an ultra-large-scale antibody library was constructed with reference to the methods provided in the following literature. The phage lysate prepared from the constructed antibody library was diluted to 10E10 with YT medium containing 7% DMSO, then divided into 1 ml aliquots, and stored at -80°C for later use.
[0083] 1. Hoogenboom HR, and G Winter, 1992, By-passing immunisation: human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J MolBiol, 227(2): 381-388.
[0084] 2. Griffiths AD, SC Williams, O Hartley, IM Tomlinson, P Waterhouse, WL Crosby, RE Kontermann, PT Jones, NM Low, TJ Allison, TD Prospero, HR Hoogenboom, A Nissim, JPL Cox, JL Harrison, M Zaccolo, E Gherardi, G Wi...
Embodiment 2
[0117] Example 2: Preparation of recombinant monoclonal antibody protein
[0118] 1. Preparation of endotoxin-free plasmid DNA: inoculate 100ml LB medium with bacteria with phCMV-II / 3K7F5L and phCMV-II / 3K7F5H plasmids, and prepare plasmid DNA with Qiagen's Ultrapure Plasmid DNA Purification Kit.
[0119] 2. Transfection and culture of CHO cells: Transfect mammalian cells with the plasmid DNA prepared above and Invitrogen's LipoFamine2000. The transfection process is carried out by the method provided by the manufacturer. Alternatives such as PEI35000 can also be used.
[0120] 3. Transfection conditions: (1) Add CHO DHFR(-) cells (ATCC No. CRL-9096) to 5ml of newly prepared Ex302 medium (purchased from Sigma-Aldrich) to a final density of 2×10E5 cells / ml. After 48 hours incubation at 37°C, the cells were collected by centrifugation at 450×g for 10 minutes. (2) Add 1ml of fresh DMEM medium, tap the bottom of the tube gently to resuspend the cells. Then, the cells were transfected w...
Embodiment 3
[0123] Example 3: Expression of Fab format and preparation of recombinant protein
[0124] (1) Construction of expression vector
[0125] The DNA fragments carrying the aforementioned SEQ ID NO: 1 and SEQ ID NO: 3 were cloned into the pCOM3H vector (Wu SC, Lin YJ, Chou JW, Lin CW. 2004, Construction and characterization of a Fab recombinant protein for Japanese encephalitis virus neutralization. Vaccine. 25, 23(2):163-71). The 5'-light chain with NheI and NotI sites was digested with NheI / NotI and ligated to pCOM3H pre-cut with the same enzyme combination. Then the heavy chain with XhoI and SpeI is digested with the same enzyme combination and then ligated to its XhoI / SpeI site. After transforming the competent cells of Escherichia coli DH5α, the single clones were tested and isolated on an agar plate containing IPTG / X-gal and ampicillin, and the selected clones were confirmed by restriction digestion. A correct clone was inoculated into 500ml LB medium containing ampicillin and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com