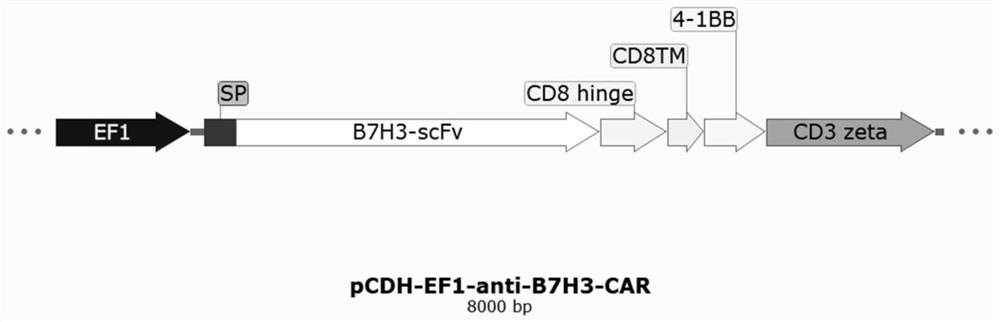
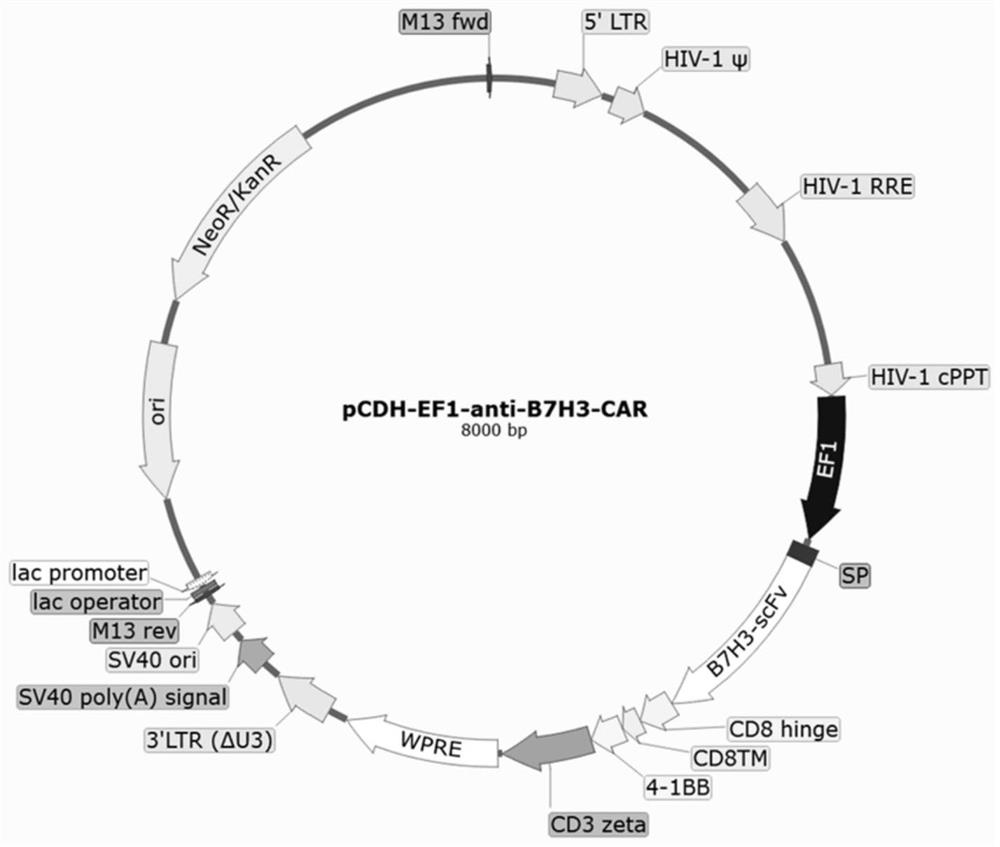
Anti-B7H3 chimeric antigen receptor and application thereof
A technology of chimeric antigen receptor and antigen, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1 Preparation of CAR-T cells
[0132] In this example, anti-B7H3 antibodies H26B6, H2B8, 26B6, 2B8, 23H1, 6F7, Enoblituzumab (Eno) and huM30 were selected as antigen-binding domains to construct CAR molecules, among which, 26B6 and its humanization H26B6, 2B8 and its humanization H2B8, 23H1, and 6F7 have significant binding ability to B7H3, not only can bind to free B7H3 protein, but also can bind to B7H3 protein on the cell surface; huM30 is a humanized B7H3 antibody (CN103687945B) of Daiichi Sankyo, Japan ), is conducting phase I clinical trials for the treatment of B7H3-positive solid tumors (NCT02192567); Enoblituzumab (MGA271) is a novel monoclonal antibody targeting B7H3 that has been optimized by immune molecules, and is developed by MacroGenics using exclusive Fc optimization technology Enoblituzumab has unique antibody advantages and therapeutic potential. There is no such drug approved in the world. Enoblituzumab represents the world's leading B7H3 ant...
Embodiment 2
[0149] Example 2 CAR-T cell expression efficiency of CAR
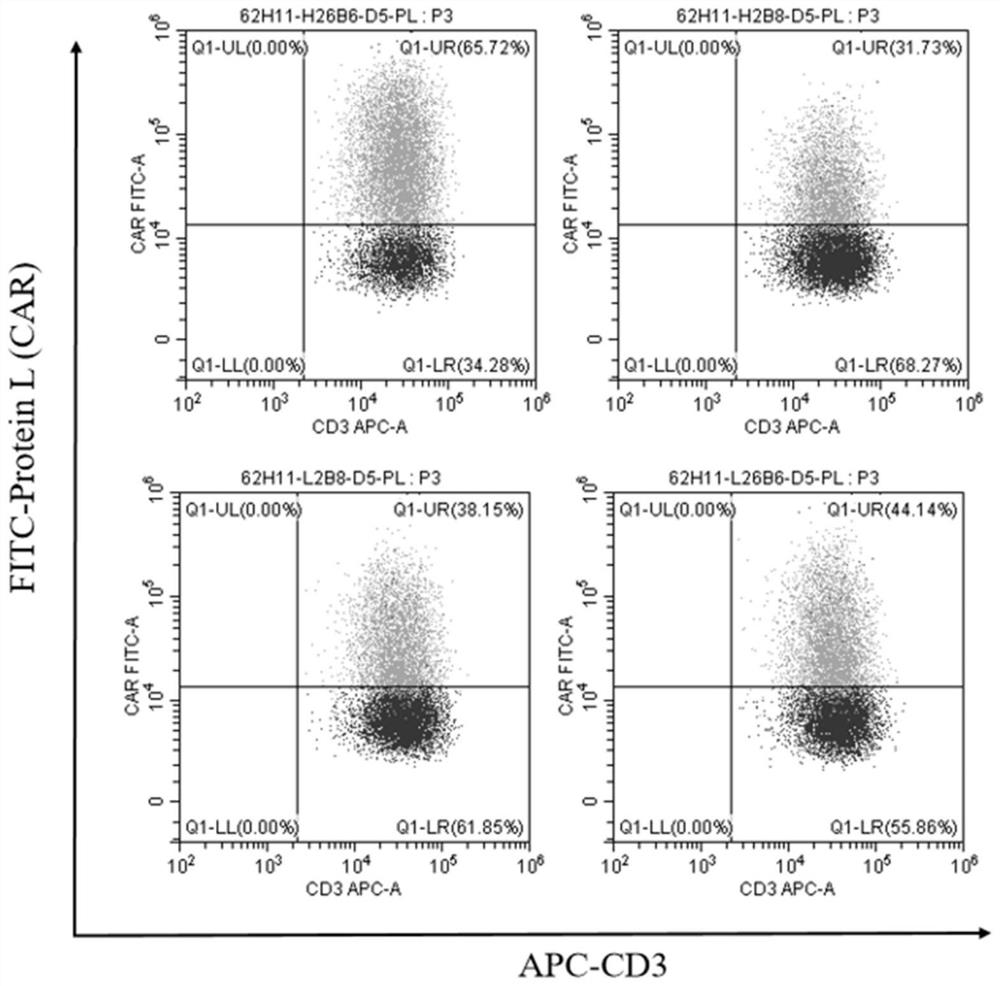
[0150] The expression rate of CAR in CAR-T cells was detected by flow cytometry.
[0151] Such as Figure 3A As shown, the expression rate of CAR in H26B6-CAR-T cells was 65.72%, and the expression rates of CAR in H2B8-CAR-T, L2B8-CAR-T, and L26B6-CAR-T cells were 31.73%, 38.15%, and 44.14%, respectively.
[0152] Such as Figure 3B As shown, the expression rate of CAR in Eno-CAR-T cells was 27.3%, and the expression rate of CAR in huM30-CAR-T cells was 45.2%.
[0153] In another experiment, as Figure 3C As shown, the expression rate of CAR in huM30-CAR-T cells was 23.09%, and the expression rate of CAR in 26B6-CAR-T cells was 7.67%;
[0154] In another experiment, as Figure 3D As shown, the CAR expression rate of huM30-CAR-T cells was 33.12%, that of L2B8-CAR-T cells was 33.43%, and that of 2B8-CAR-T cells was 12.55%.
Embodiment 3
[0155] Example 3 Killing function of CAR-T cells
[0156] H26B6-CAR-T, H2B8-CAR-T, L2B8-CAR-T, L26B6-CAR-T were mixed with human liver cancer cell HepG2, human pancreatic cancer cell PL45, and human cervical cancer cell SiHa at 2:1, 1:1 , 1:4 effect-to-target ratio and incubated for 16 hours, using RTCA technology to detect the killing efficiency of CAR-T.
[0157] Figure 4A , Figure 4B , Figure 4C The results showed that the four CAR-T cells had killing effects on the three tumor cells under different effect-to-target ratios, and the greater the effect-to-target ratio, the stronger the killing ability; when the effect-to-target ratio was 2:1, H26B6-CAR-T The killing efficiency of T on the three tumor cells was better than that of H2B8-CAR-T, L2B8-CAR-T and L26B6-CAR-T.
[0158] Different CAR-T cells (huM30-CAR-T and 2B8-CAR-T) were prepared using PBMCs from healthy donors (donor 1 and donor 2), respectively, and the target cells were mixed at a ratio of 3:1, 1:1, and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com