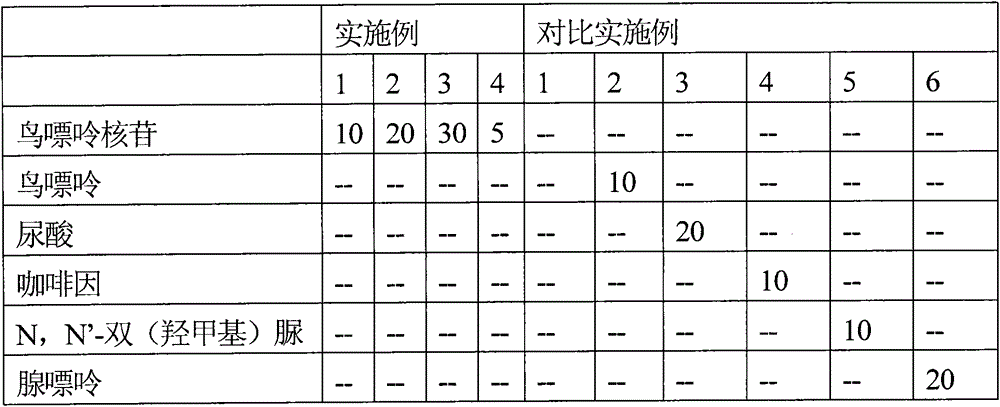
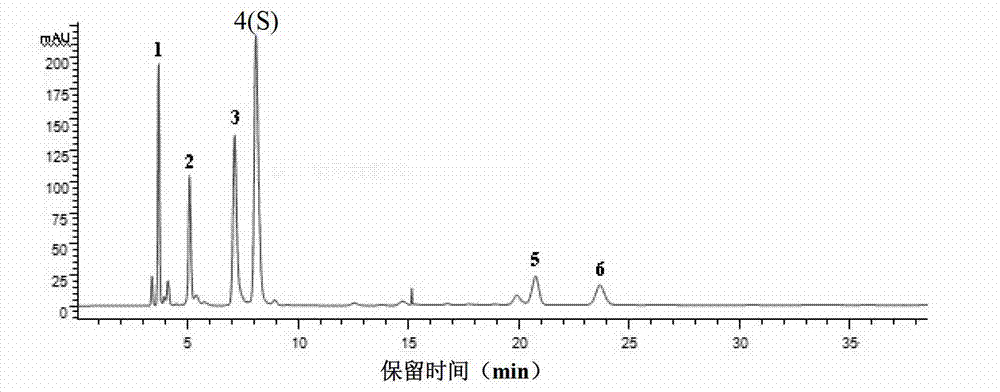
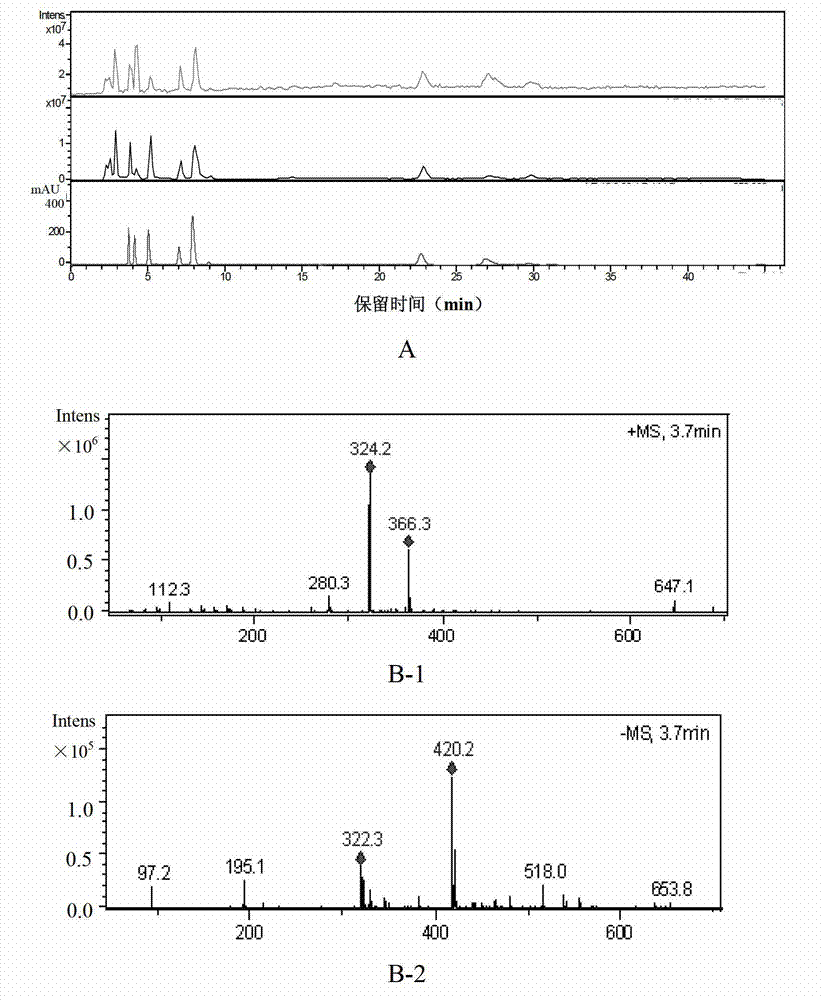
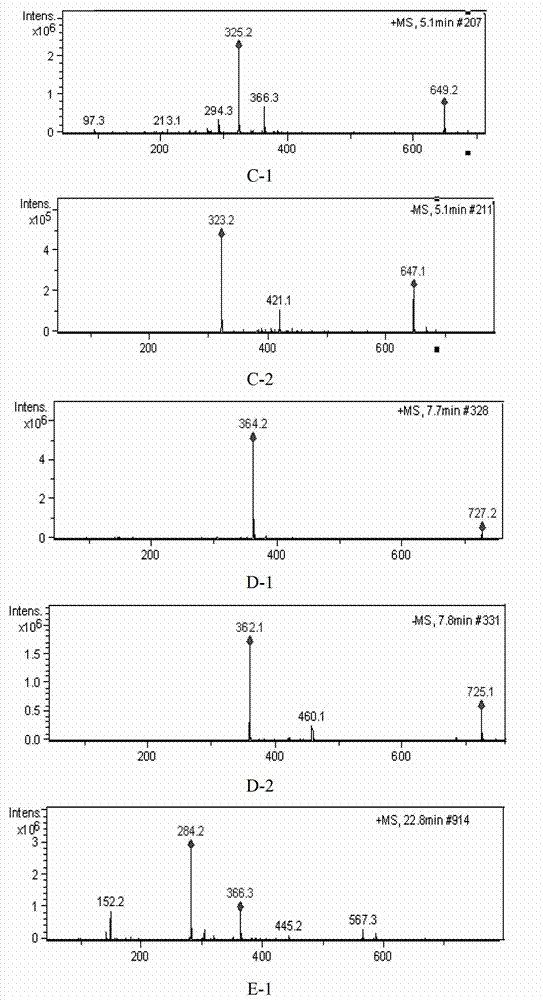
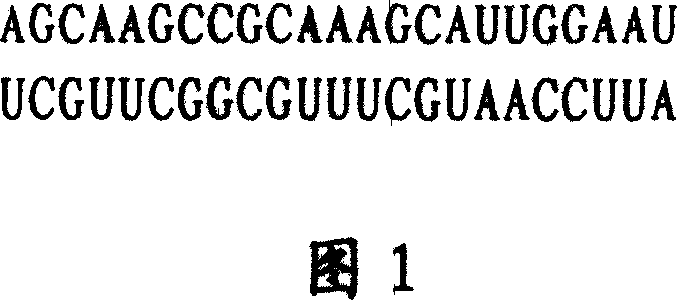Patents
Literature
65 results about "Guanine nucleoside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
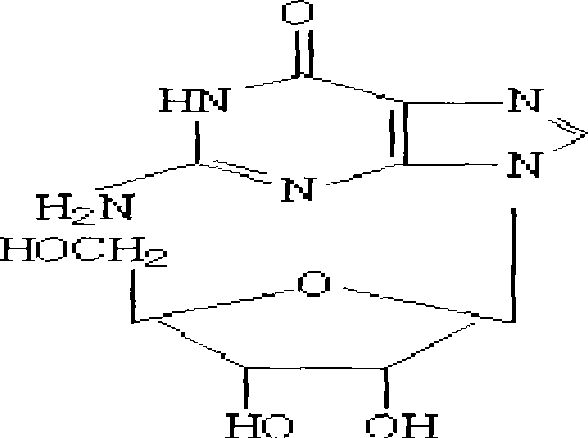
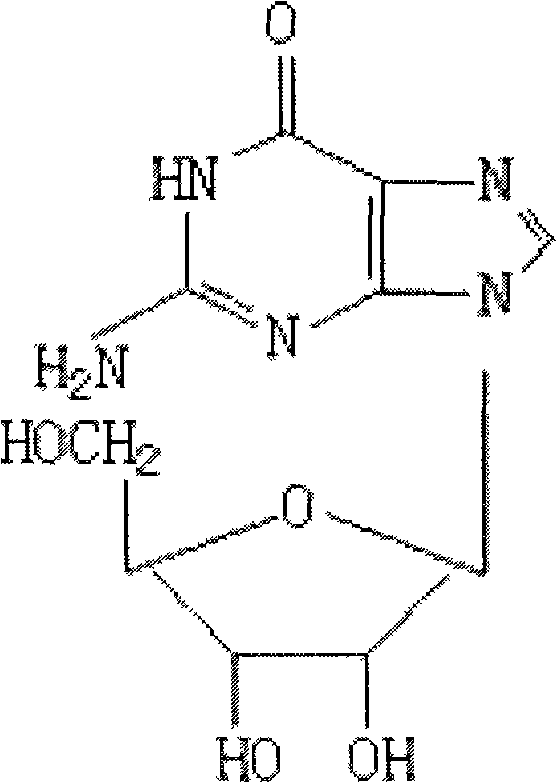
Condensation product of a guanine base plus a sugar.
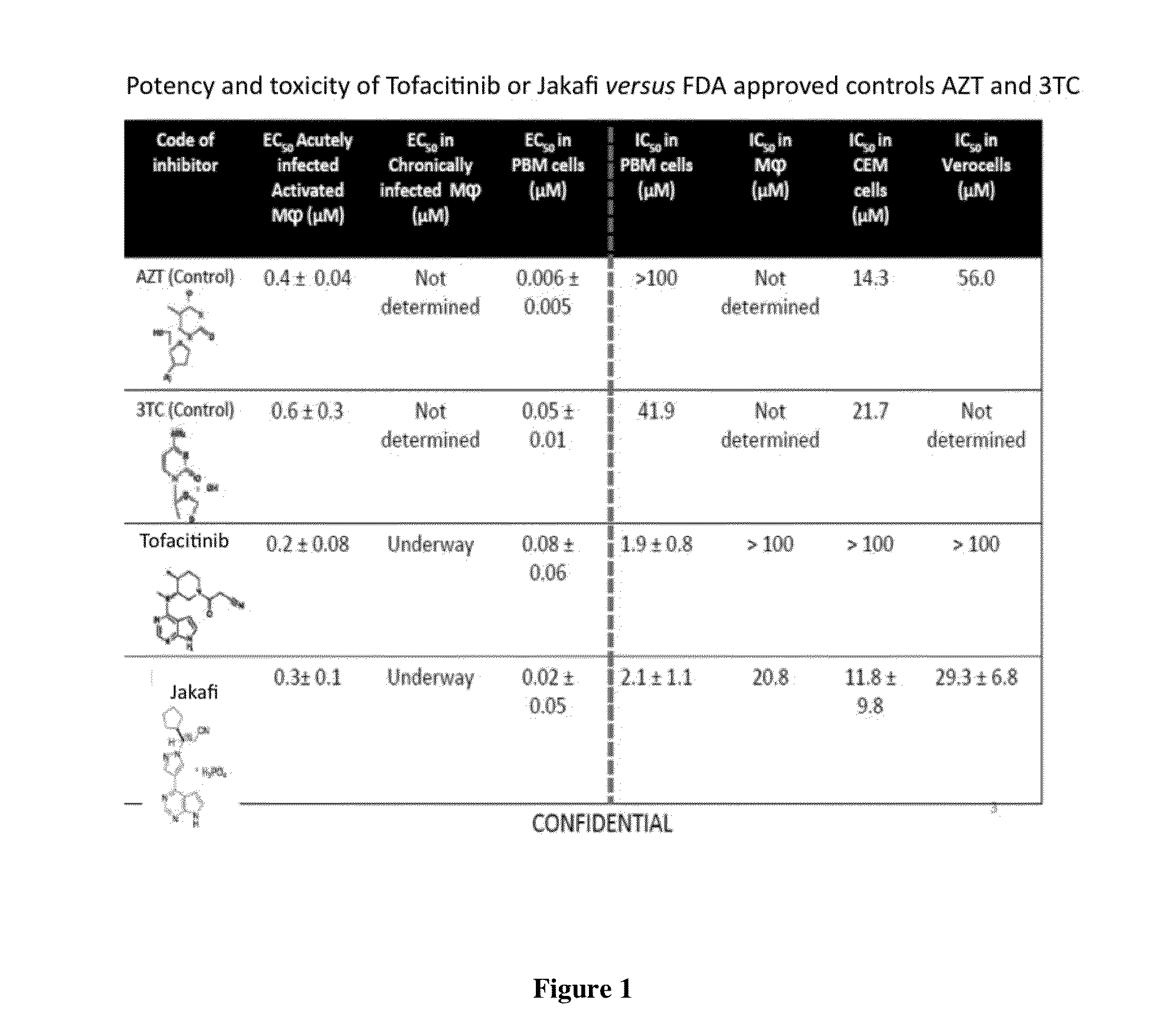
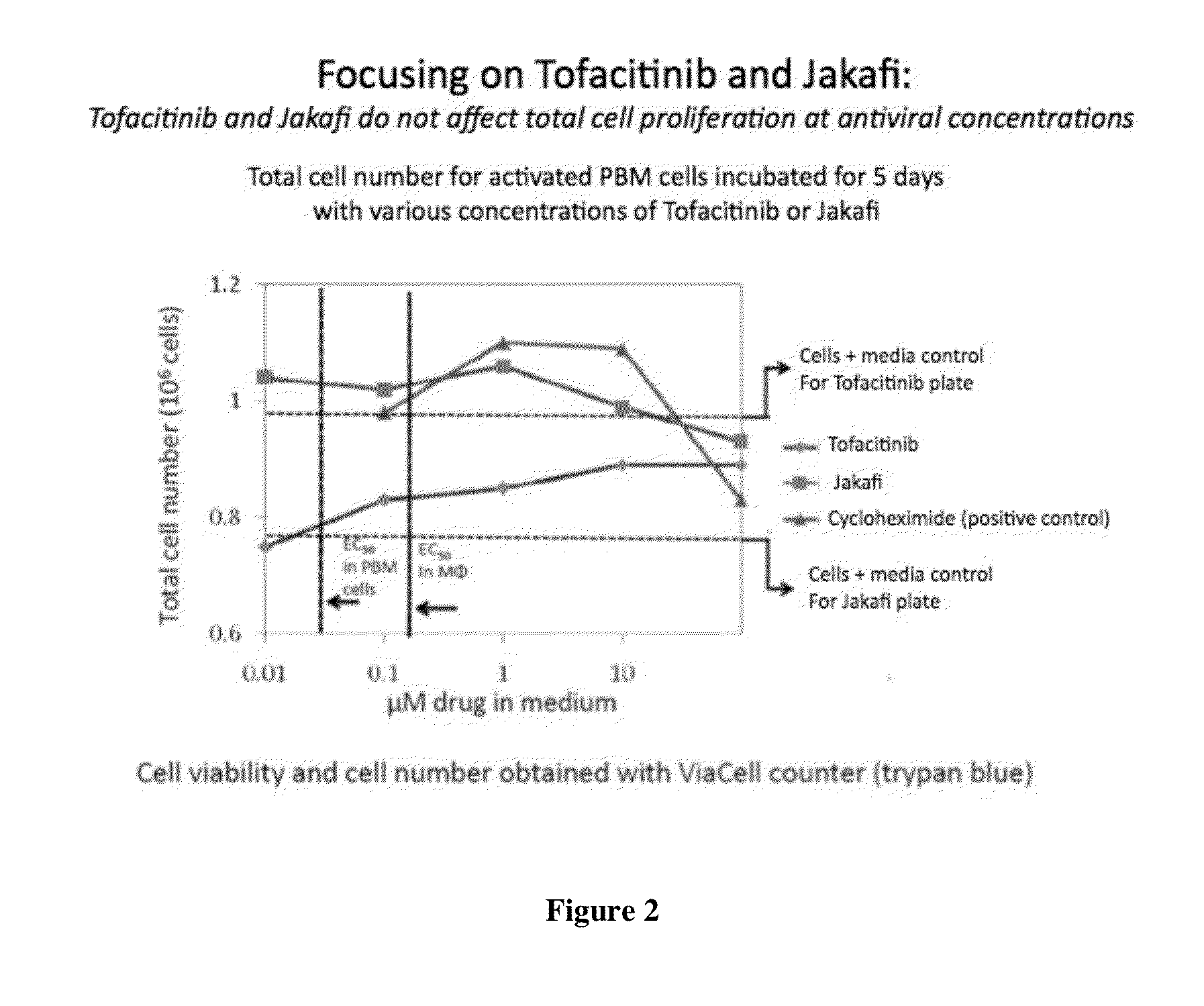
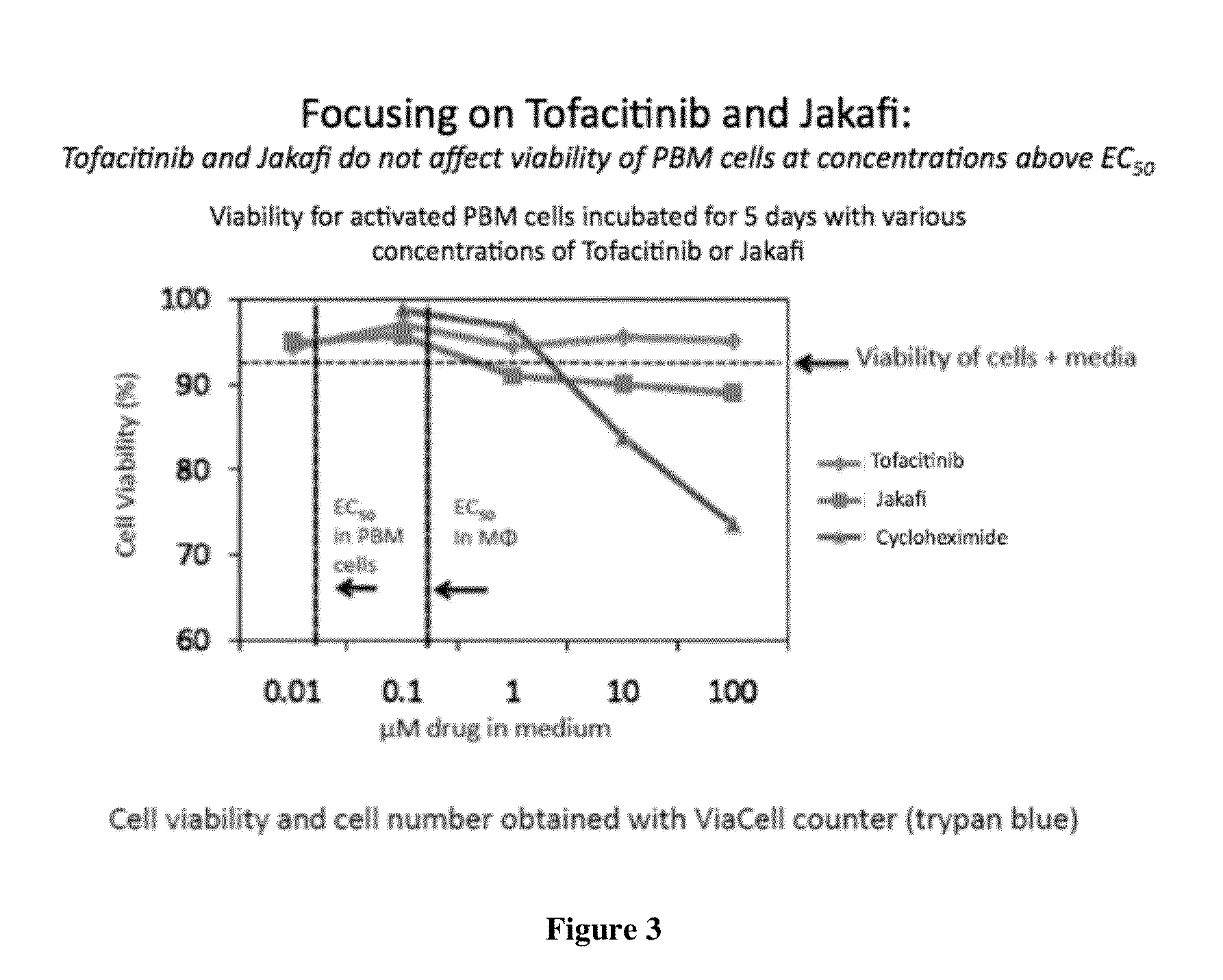
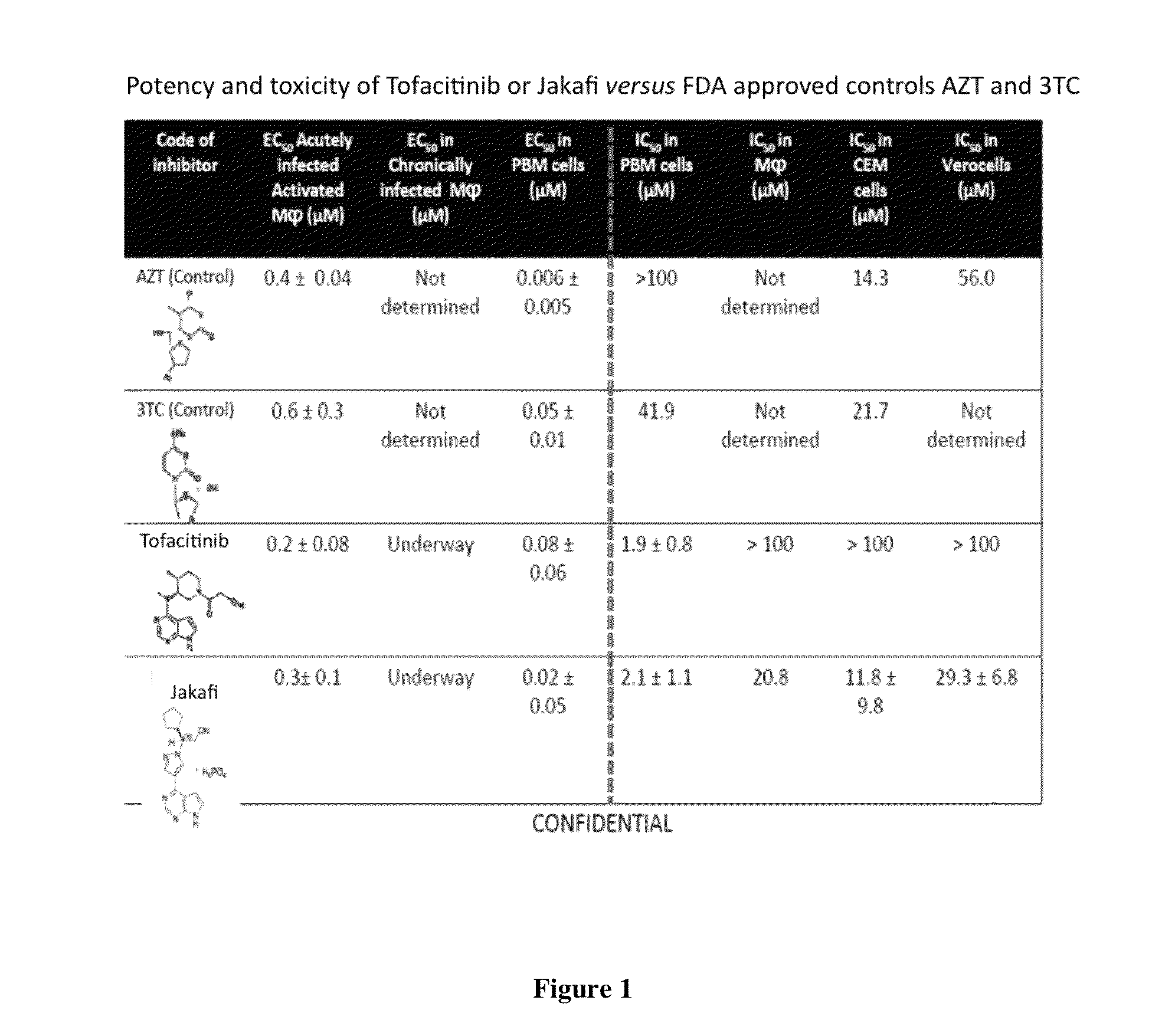
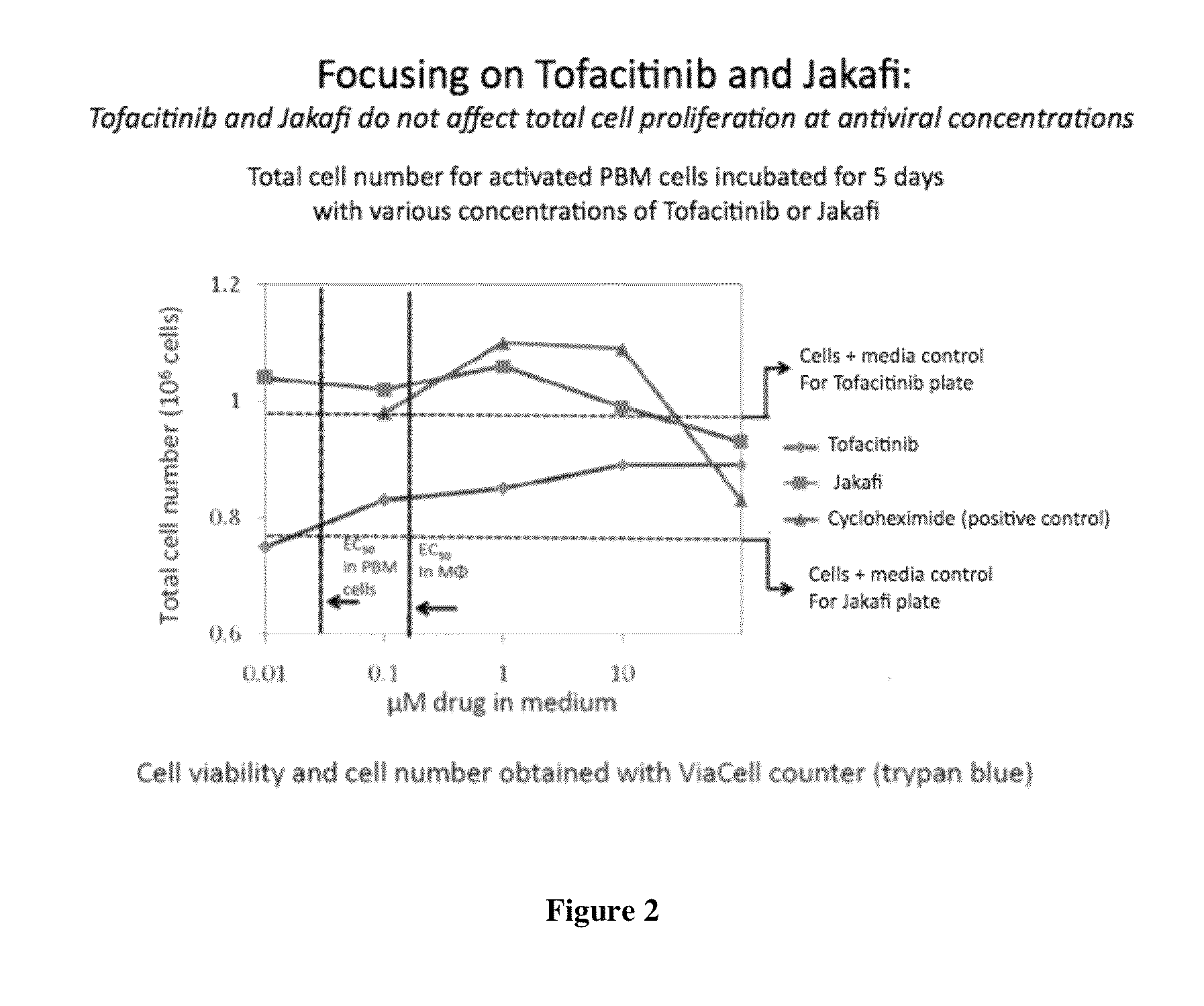
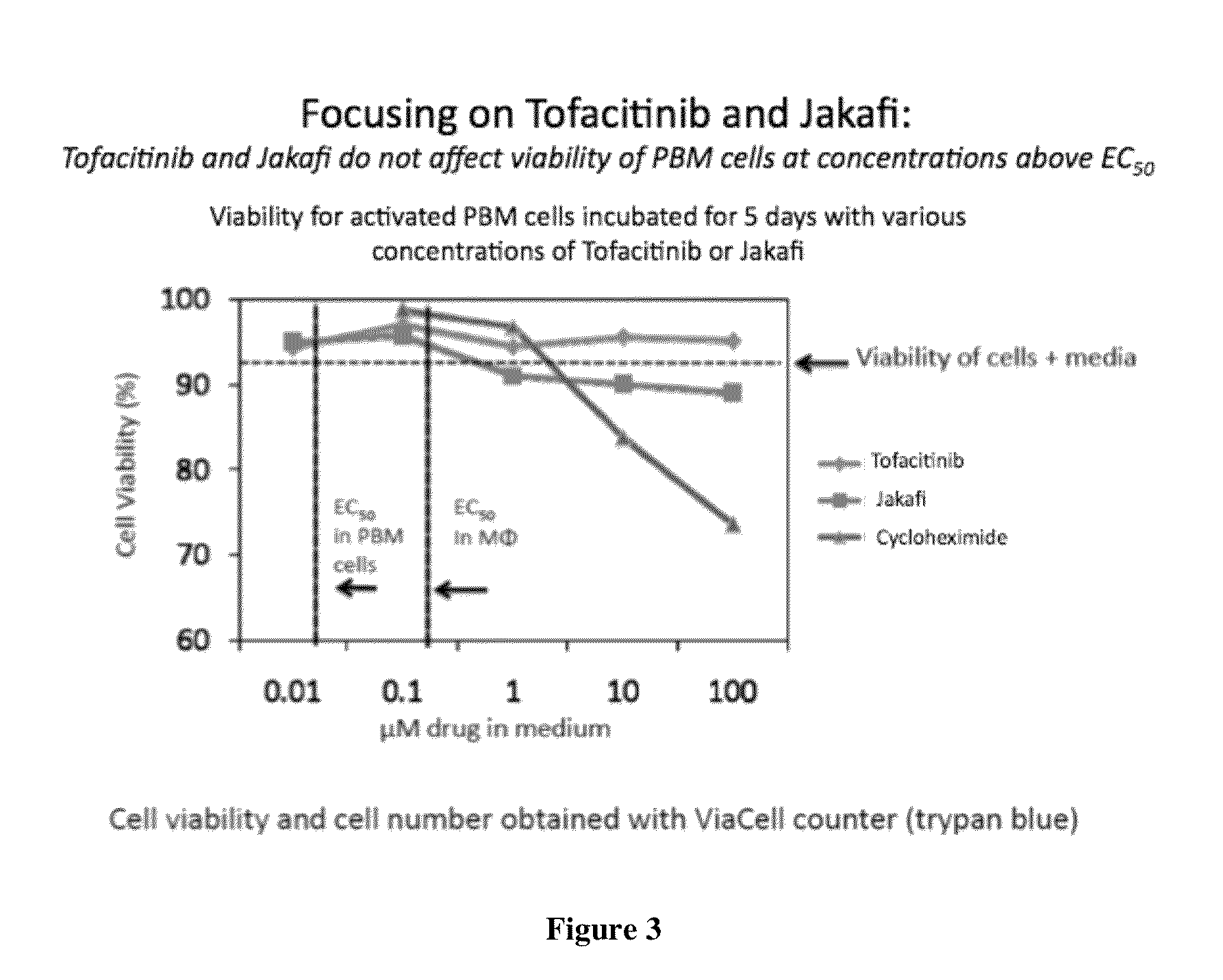
Antiviral jak inhibitors useful in treating or preventing retroviral and other viral infections
ActiveUS20140328793A1Improve their absolute antiviral effectLow toxicityBiocidePeptide/protein ingredientsProteinase inhibitorThymidine
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
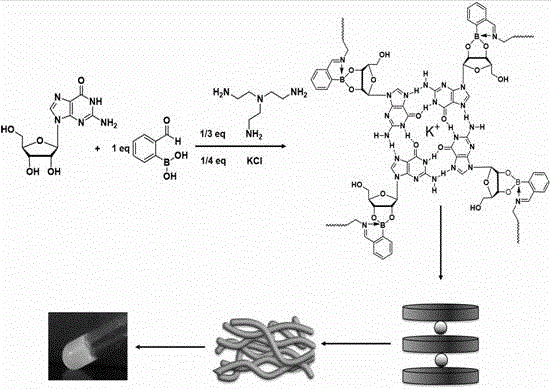
Sugar response supramolecular gel with G-quadruplex structure and preparation method thereof
InactiveCN105622692AHigh strengthSugar responsiveOrganic active ingredientsSugar derivatives2-formylphenylboronic acidRaw material
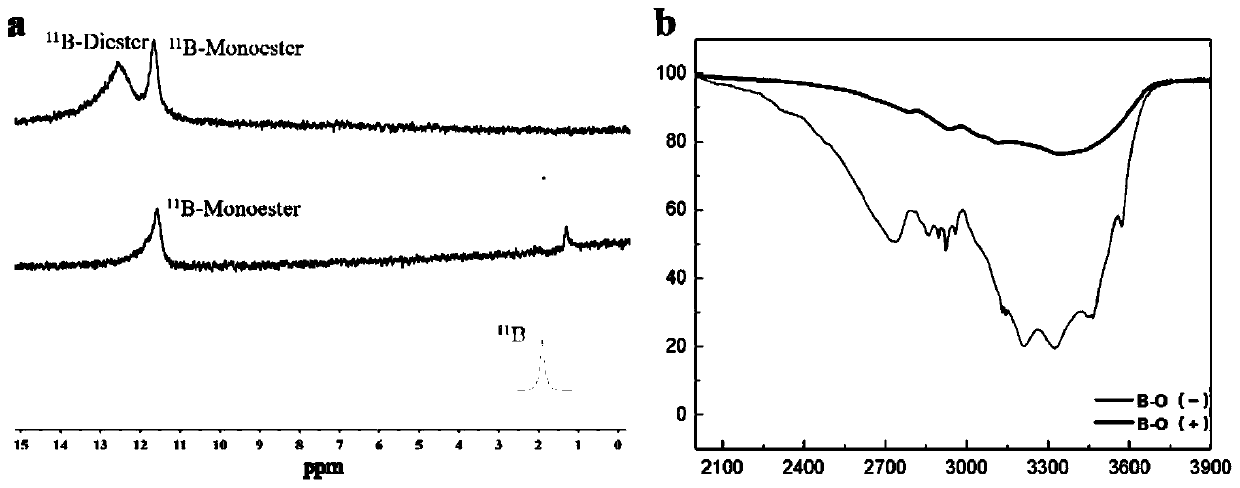
Disclosed is sugar response supramolecular gel with a G-quadruplex structure. A preparation method includes: taking sugar response of 2-formylphenylboronic acid as a center; utilizing a vicinal diol structure that vernine has to react with the 2-formylphenylboronic acid to form dynamic covalent bond boron ester bond; enabling primary amine in tri(2-amino ethyl)amine and aldehyde group of the 2-formylphenylboronic acid to form dynamic imine bond; forming the G-quartet structure when potassium ions among basic groups of vernine are stable. The sugar response supramolecular gel can be used for detecting release effect of methylthionine chloride in a glucose solution and an acidic solution. The sugar response supramolecular gel and the preparation method have the advantages that the gel prepared by the method is stable, high in strength, high in sugar response performance and capable of loading a lot of macromolecular / micromolecular gel; raw materials related to the preparation method are simple, and the supramolecular gel can be prepared by utilizing micromolecules while complex synthesis steps are not needed, so that cost is low, production process is simple, products can be stored for a long time without going bad, the raw materials are low in toxicity, and the preparation method is easy to popularize and apply.
Owner:NANKAI UNIV
Chiral supramolecular nucleoside hydrogel based on boron ester bonds as well as preparation method and application of chiral supramolecular nucleoside hydrogel
ActiveCN111533926AImprove stabilityGood injectabilityPharmaceutical delivery mechanismCell culture supports/coatingCell-Extracellular MatrixGuanine nucleoside
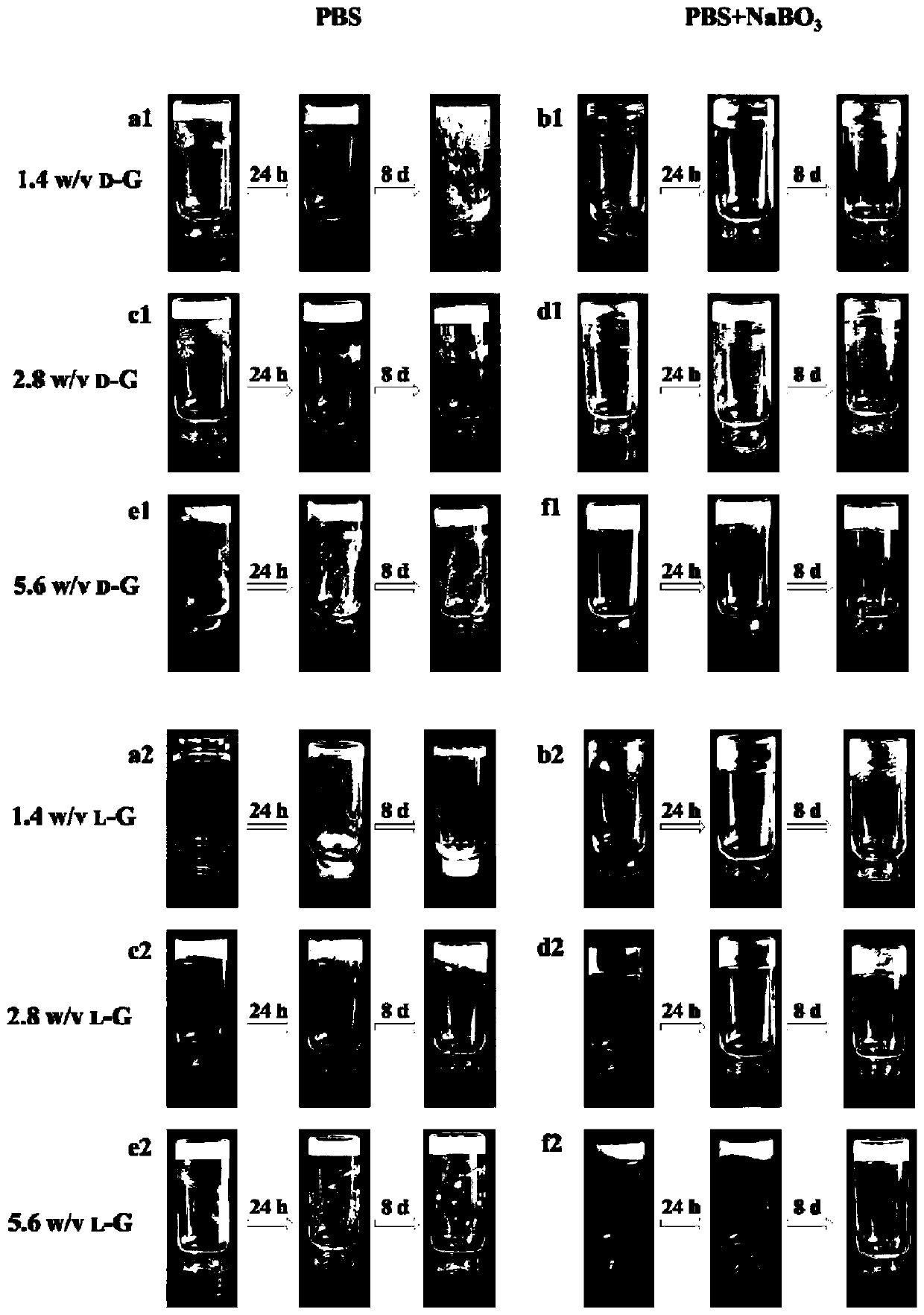
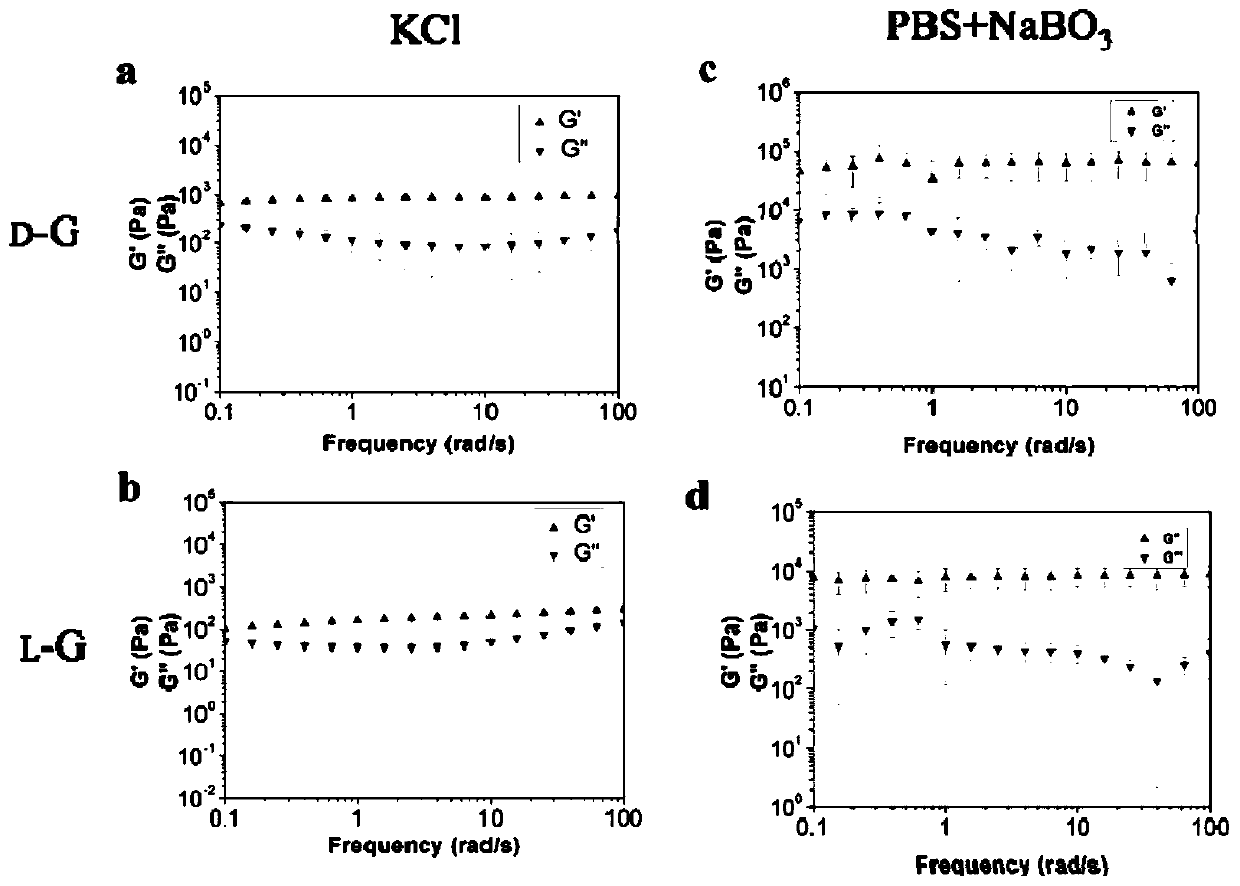
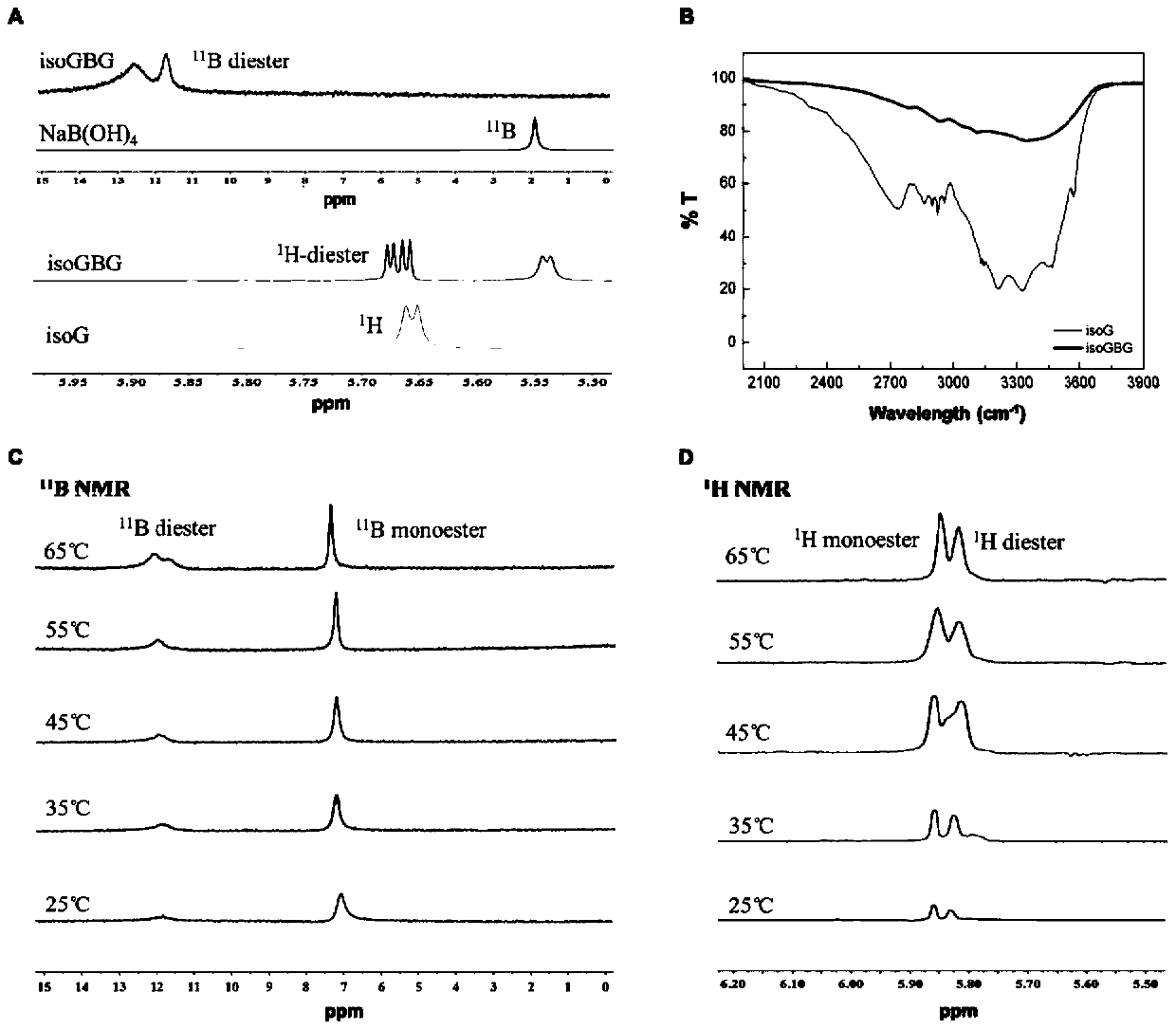
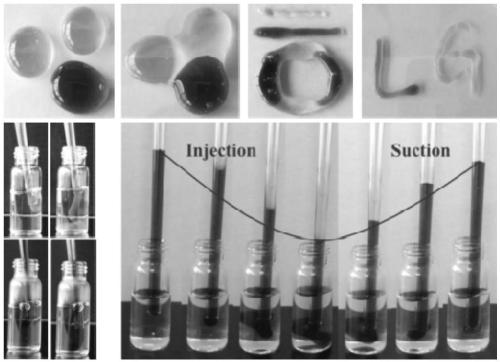
The invention relates to chiral supramolecular nucleoside hydrogel based on a boron ester bonds as well as a preparation method and application of the chiral supramolecular nucleoside hydrogel, and particularly provides supramolecular hydrogel which is obtained by mixing guanine nucleoside and borate as raw materials in a solvent, wherein the guanine nucleoside is D-guanine nucleoside and / or L-guanine nucleoside. The supramolecular hydrogel has excellent stability, injectability and self-repairability, also has good biocompatibility, does not show obvious acute toxicity in animal bodies, can be degraded in vivo, and can be used as an extracellular matrix for 3D culture of cells. The supramolecular hydrogel provided by the invention has a very good application prospect in the field of tissue engineering as a scaffold material.
Owner:SICHUAN UNIV
Synthetic circulation production process for guanine nucleoside
InactiveCN101492484AEfficient use ofIn line with the new concept of green environmental protectionSugar derivativesAnimal feeding stuffFiltration membraneUltrafiltration
The invention provides a comprehensive recycling production process of guanosine. The bacterial suspension collected from the process in which guanosine is extracted and refined from guanosine fermented liquid is dried as feed additive or made into feed protein; the primary mother liquid acquired from the first four times during micro-filtrate membrane filtration, crude crystallization and pressure filtration and separation is sent into a reverse osmosis membrane for condensation and then mixed with the guanosine fermented liquid for internal recycling; the primary mother liquid acquired from the fifth time undergoes evaporation concentration, crystallization, drying and granulation to produce ammonium sulfate and other bio-compound fertilizers; the secondary mother liquid acquired from ultrafiltration membrane treatment is reused as micro-filtration membrane dialysis water; the third mother liquid acquired from extract crystallization and pressure filtration and separation is respectively reused as anti-crystallinic water of anti-crystallinic jar and dialysis water of micro-filtration membrane and ultrafiltration membrane. The invention overcomes the defects of the traditional process such as incomprehensive utilization, resources waste and environmental pollution; the comprehensive cycling process is reasonable and feasible and conforms to the green environmental protection concept, thus cleaning the environment, promoting the ecological balance, reducing the production cost and strengthening the social and economic benefits.
Owner:湖南汉晶瑞氨基酸有限公司
Bifunctional nucleoside hydrogel and preparation method and application thereof
InactiveCN110151776AEnhanced inhibitory effectOrganic active ingredientsSugar derivativesDihydrogen oxideSquamous Carcinomas
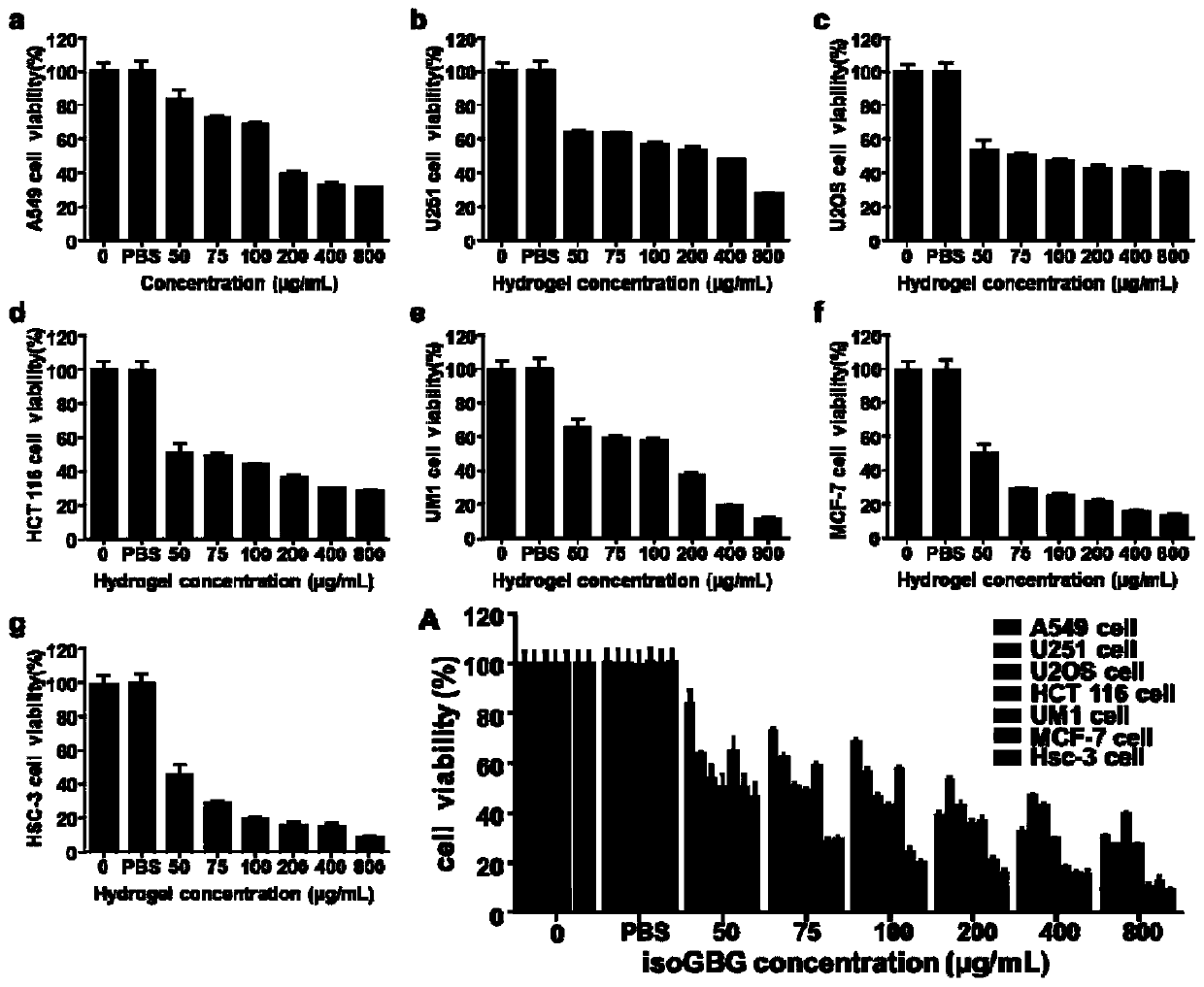
Disclosed is a bifunctional nucleoside hydrogel. The bifunctional nucleoside hydrogel is formed by dissolving isoguanosine, guanosine and borate in water or an aqueous solution for crosslinking. The bifunctional nucleoside hydrogel integrates a carrier and a drug effect and has an obvious inhibitory effect on the activity of tumor cells, in particular to an obvious inhibitory effect on the activity of cells related to lung cancers, gliomas, osteomas, colon cancers, breast cancers, oral squamous cell carcinomas and tongue squamous carcinomas. The inhibitory effect on the activity of the oral squamous cell carcinoma cells is the best. In addition, the bifunctional nucleoside hydrogel can inhibit the growth of HSC-3 transplanted tumors of the oral squamous cell carcinoma cells in vivo. Therefore, the bifunctional nucleoside hydrogel has potential application prospects in the aspect of preparing anti-tumor drugs. Particularly, a new way can be provided for treating the oral squamous cell carcinomas.
Owner:SICHUAN UNIV
Self-repairing supermolecule hydrogel as well as preparation method and use thereof
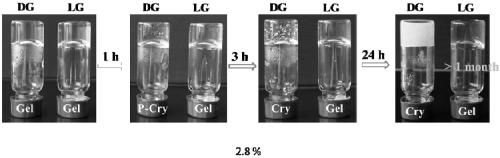
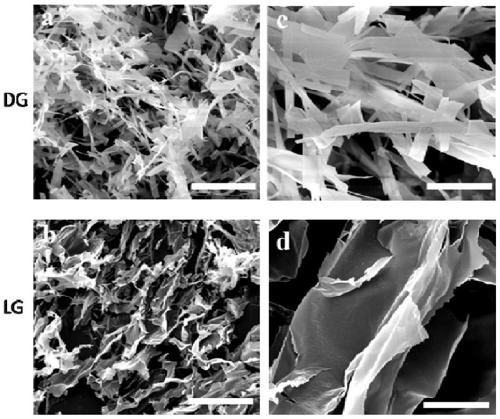
ActiveCN109180963AOvercome the defect of easy crystallization instabilityExcellent self-healing performanceGuanine nucleosideBiocompatibility Testing
The invention provides self-repairing supermolecule hydrogel as well as a preparation method and use thereof. Particularly, the supermolecule hydrogel is prepared by the steps of dissolving L-type guanosine into a potassium chloride water solution, heating until boiling, and cooling at the room temperature after L-type guanosine is completely dissolved, so as to obtain the supermolecule hydrogel.Experimental results prove that the supermolecule hydrogel formed through the self-assembling of L-form guanosine has the advantages that the defects of D-type guanosine hydrogel are overcome, and thesupermolecule hydrogel presents very good self-repairing capacity and biocompatibility and has very good application prospects in the field of biological medicine.
Owner:SICHUAN UNIV
Production method of guanine nucleosides
InactiveCN101487036AGenetic stabilitySynchronous productivity is highMicroorganism based processesFermentationUltrafiltrationMicrofiltration membrane
The invention discloses a guanosine production method, which consists of four major technical processes including fluid strain cultivation in a shaking bottle, production of glucose solution, production of guanosine fermented fluid and extraction and refinement of guanosine, adopts a preserved strain TA208-IMPD with genetic stability, prepares the glucose solution by steeping, pulping, liquefying, filter pressing and saccharifying cracked grains, adopts new techniques including flow feeding of the glucose solution in a fermentation process, and produces qualified guanosine through microfiltration membrane sterilization, crude guanosine crystallization, ultrafiltration membrane decoloring and impurity removing, separating and parching of the guanosine fermented fluid. The guanosine production method overcomes the disadvantages of traditional new techniques that have behindhand culture techniques, lower guanosine yield of the strain, long fermentation period, lower transformation ratio and lower synthesized extraction yield, and the like, achieves high synchronized production efficiency and guanosine production level of the strain, stable glucose solution product and high guanosine fermented production level, and can reach the guanosine product purity of over 99 percent and the yield of over 80 percent.
Owner:湖南赛康德生物科技有限公司
Antiviral JAK inhibitors useful in treating or preventing retroviral and other viral infections
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
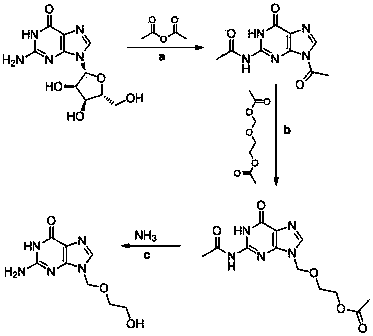
Preparation method of acyclovir
InactiveCN103664944ARaw materials are cheap and easy to getStable sourceOrganic chemistrySimple Organic CompoundsBiochemical engineering
The invention discloses a preparation method of acyclovir, and belongs to the field of organic compound synthesis. According to the method, guanosine is taken as a starting raw material and is subjected to reactions of acylation, condensation and hydrolysis to prepare the acyclovir. Compared with the prior art, the synthesis method has stable and abundant raw material sources, market fluctuation influences are small, reaction conditions are mild, the operation is simple and safe, the reaction yield is high, the production cost is low, three wastes are few, and greater implementation value and socioeconomic benefits are provided.
Owner:XINXIANG WEIDE CHEM CO LTD
Supramolecular nucleoside hydrogel and preparation method and application thereof
ActiveCN113058076AImprove stabilityGood injectabilityPharmaceutical non-active ingredientsCoatingsDiseaseTopical treatment
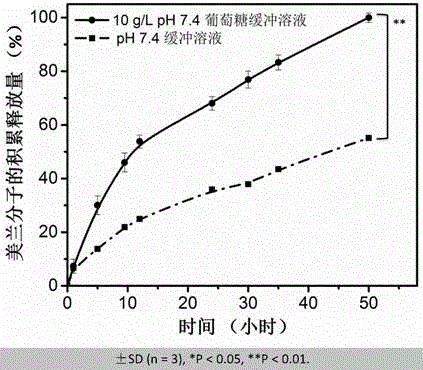
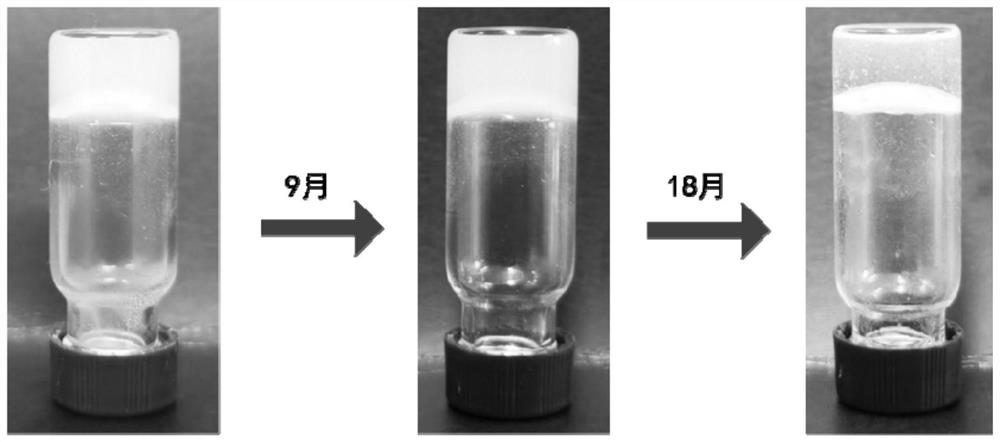
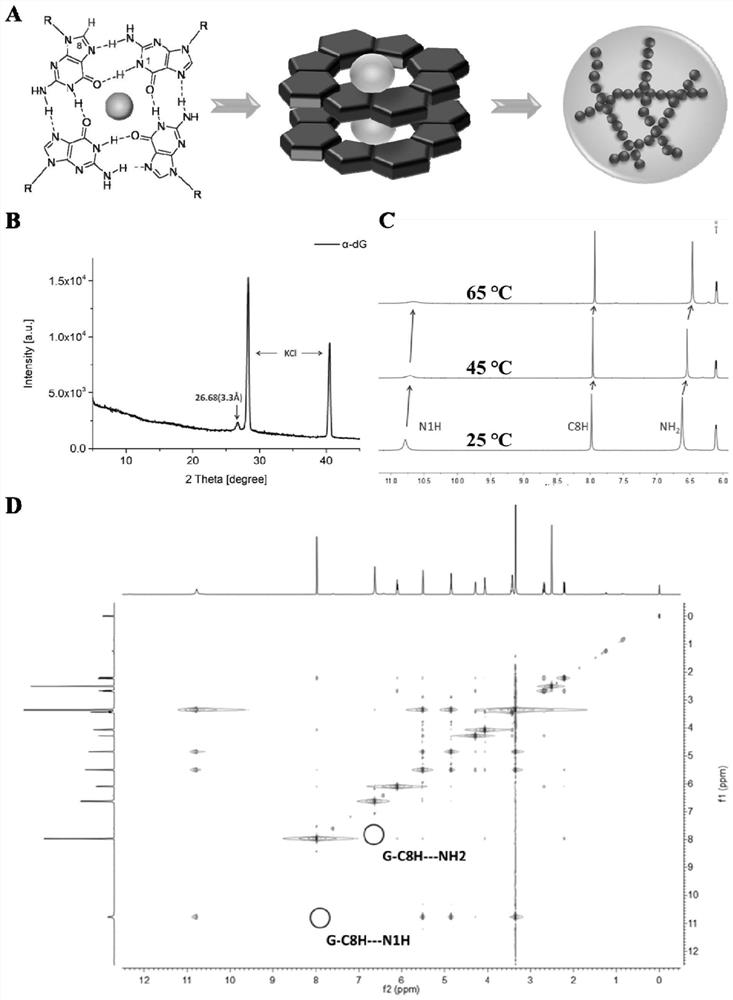
The invention provides supramolecular nucleoside hydrogel and a preparation method and application thereof, and belongs to the field of biomedical materials. In a powder X-ray diffraction pattern of the hydrogel, diffraction peaks exist at 2theta diffraction angles of 26.7 degrees, 28.4 degrees and 40.5 degrees. The supramolecular hydrogel based on D-configuration alpha-deoxyguanosine is successfully constructed, the alpha-dG hydrogel has excellent stability and cannot be disintegrated and damaged after being placed for 18 months, and the problem that in the prior art, supramolecular hydrogel formed by D-type guanosine is poor in stability is solved. Besides, the alpha-dG hydrogel has good injectability, biocompatibility and drug delivery capacity, can be used as a drug carrier, and is expected to be further applied in the field of biomedicine, such as local treatment of oral mucosa diseases.
Owner:SICHUAN UNIV
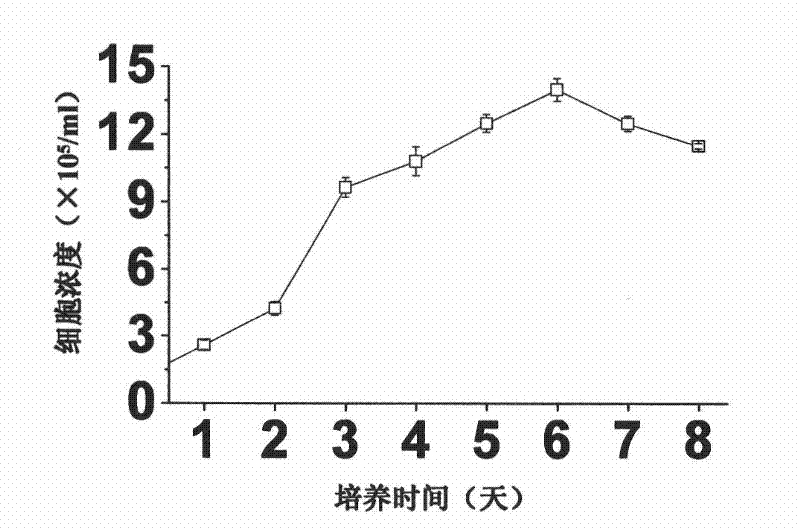
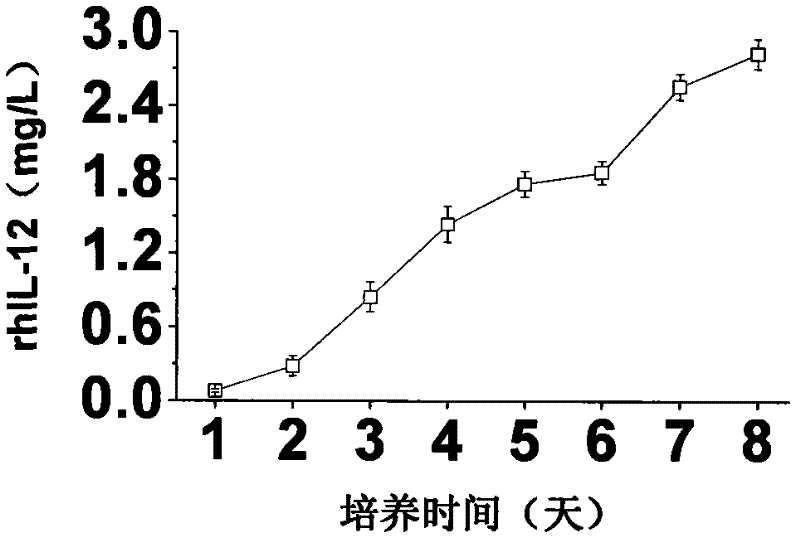
A large-scale serum-free culture method of rhil-12 engineered cells
The invention relates to a large-scale serum-free culture method for rhIL-12 engineering cells, which comprises the following step of: inoculating rhIL-12 engineering cells in a logarithmic phase into a serum-free and protein-free medium for culture, wherein the medium is a CD CHO liquid medium comprising sodium pyruvate at the final concentration of 0.8 to 1.2mM, hypoxanthine at the final concentration of 0.075 to 0.125mM, thymidine at the final concentration of 0.012 to 0.020mM, adenosine at the final concentration of 0.5 to 0.9mg / L, guanosine at the final concentration of 0.5 to 0.9mg / L, cytidine at the final concentration of 0.5 to 0.9mg / L, uridine at the final concentration of 0.5 to 0.9mg / L, L-glutamine at the final concentration of 0.4 to 0.8mg / L, L-asparagine at the final concentration of 0.4 to 0.8mg / L, L-proline at the final concentration of 1.5 to 2.0mg / L and non-essential amino acid at the final concentration of 0.08 to 0.125mM. The invention also provides a medium used in the method. By the medium and the culture method, a high-yield and high-activity recombinant human interleukin-12 can be obtained.
Owner:UNIV OF SCI & TECH OF CHINA
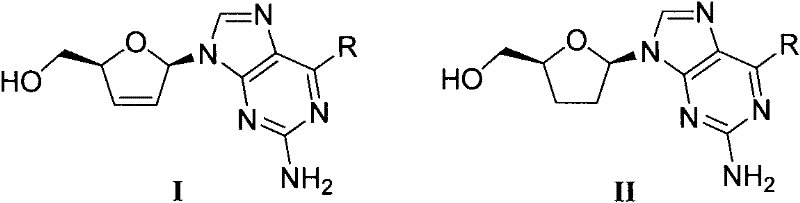
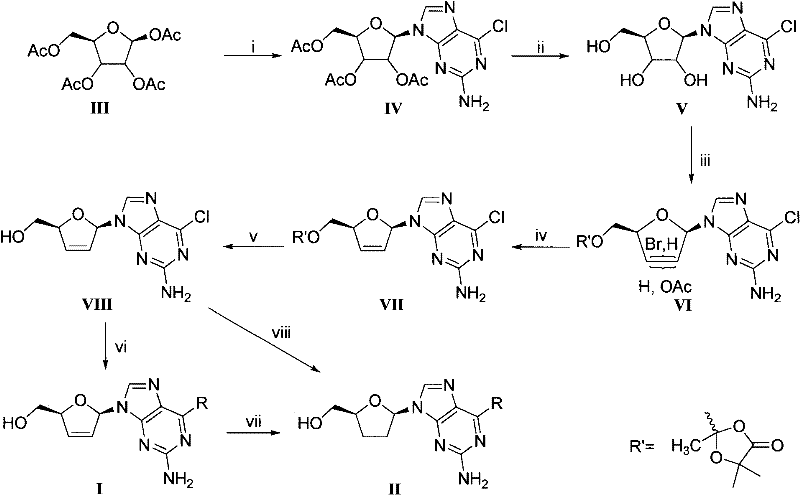
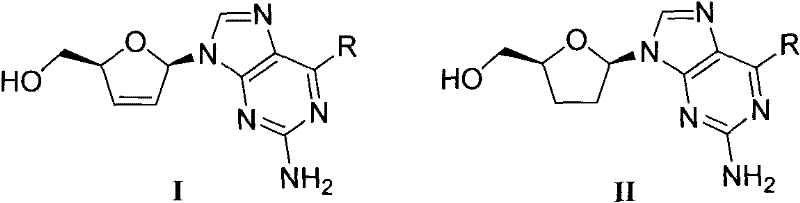
D, L-guanosine analogs, preparation methods thereof and applications thereof
The invention discloses D, L-guanosine analogs, preparation methods thereof and applications thereof. The structural formulas of the analogs are respectively represented by a general formula I or a general formula II, wherein R is hydrogen, amino, piperidyl, morpholinyl or pyrrolidinyl. The good chemical stability and the pharmacokinetic stability showed by the D, L-guanosine analogs of the invention make up for shortages of low stability, poor pharmacokinetic stability and the like existing in D4G and ddG. The preparation methods of the D, L-guanosine analogs have the advantages of mild reaction, simplicity and high yield. Antiviral activity testing shows that the compounds with the general formula I or the general formula II which have excellent antiviral activity can be applied to prepare anti-HIV drugs, anti-HBV drugs, anti-HCV drugs or anti-HSV drugs.
Owner:PEKING UNIV
Modified nucleosides and nucleotides and uses thereof
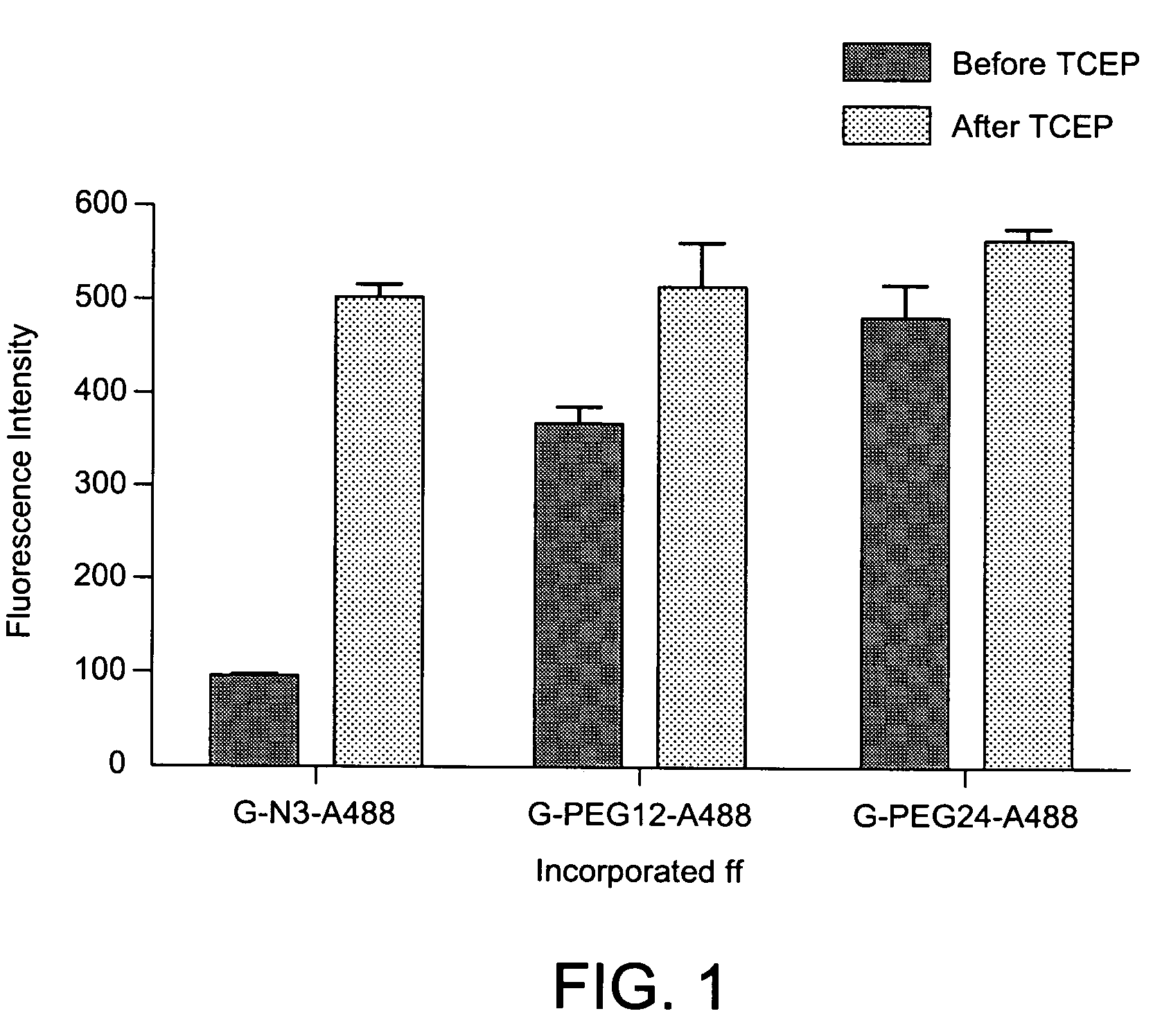
ActiveUS20070042407A1High fluorescence intensityIncrease brightnessSugar derivativesMicrobiological testing/measurementGuanine nucleosidePurine
The invention is directed to modified guanine-containing nucleosides and nucleotides and uses thereof. More specifically, the invention relates to modified fluorescently labelled guanine-containing nucleosides and nucleotides which exhibit enhanced fluorophore intensity by virtue of reduced quenching effects.
Owner:ILLUMINA CAMBRIDGE LTD
Electroless copper plating solution
InactiveCN104372315AGood lookingIncrease stickinessLiquid/solution decomposition chemical coatingCopper platingGuanine nucleoside
Provided is an electroless copper plating solution that forms a highly adhesive conductive film regardless of the degree of roughness of the resin surface and also has a fast deposition rate. The electroless copper plating solution of the present invention is characterized in that it contains guanosine. The electroless copper plating solution of the present invention preferably also contains copper ion, reducing agent, copper ion complexing agent, and pH adjuster.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
Quality control method for ribonucleic acid II for injection
ActiveCN102818867AHigh technology contentImprove securityComponent separationInternational marketHydrolysis
The invention discloses a quality control method for ribonucleic acid II for injection. The method comprises the following steps that the nucleic acid enzyme hydrolysis solution of a substance to be measured is subjected to high-performance liquid chromatogram analysis, an adopted chromatographic column is an Agilent ZORBAX SB-AQC18 chromatographic column, and a flowing phase is a mixture of a formic acid solution and an acetonitrile solution; after the analysis, if the substance to be measured is determined to contain five substances as follows: cytidylate, uridine monophosphate, guanine nucleotide, guanosine and adenosine, the substance to be measured is the ribonucleic acid II for injection or is the ribonucleic acid II for injection as a candidate; and if not, the substance to be measured is not the ribonucleic acid II for injection or is not the ribonucleic acid II for injection as the candidate. A high-performance liquid chromatographic technique is utilized, and the strong-specificity quality control method for the ribonucleic acid II for injection is established. The method has important meanings on increasing the technological content of the medicine, increasing the safety and effectiveness, reducing the cost, enlarging the production scale, increasing the market occupancy, and going forward to the international market.
Owner:JILIN AODONG PHARMACEUTICAL INDUSTRY GROUP YANJI CO LTD
Gel dressing based on G-quadruplex/collagen, as well as preparation and application of gel dressing
ActiveCN106620815AGood biocompatibilityNot easy to scabAbsorbent padsBandagesSide effectPolymer science
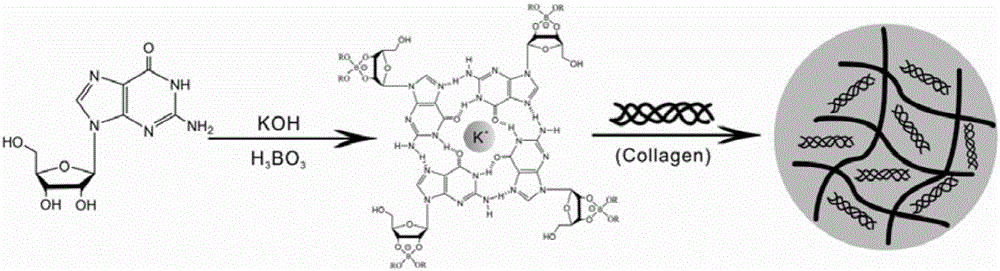
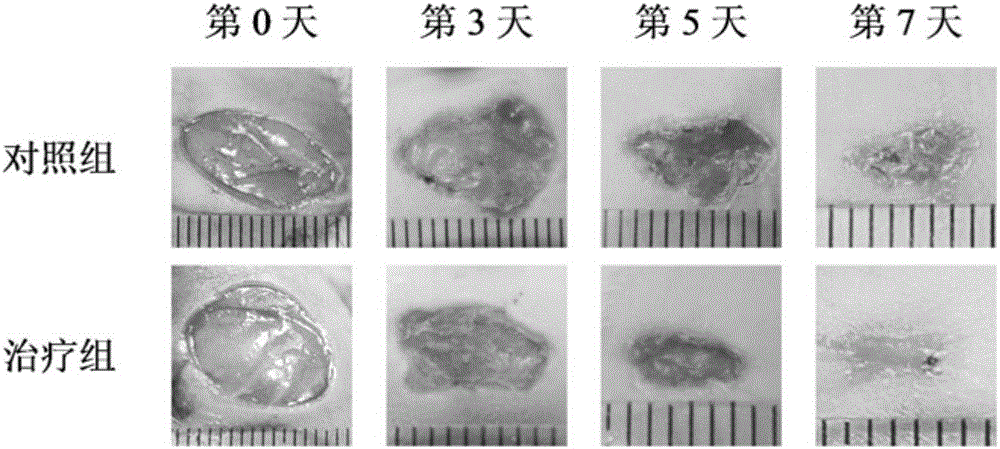
The invention discloses a gel dressing based on G-quadruplex / collagen and a preparation method of the gel dressing. The preparation method comprises the following steps: adding guanine nucleoside, boric acid and potassium hydroxide to deionized water according to the proportion of the guanine nucleoside, to the boric acid to the potassium hydroxide being (1-3):1:1 and performing heating to 90-98 DEG C to obtain a G-quadruplex solution; cooling the G-quadruplex solution to 35-40 DEG C, adding collagen to disperse the solution till the concentration of the collagen in the solution is 2-10mg / mL, and performing cooling to room temperature so as to obtain the gel dressing based on G-quadruplex / collagen. The preparation method has the advantages of simple reaction conditions, few synthesis steps, short time and low production cost. When being used for wound healing, the gel dressing has good biocompatibility, good air permeability, strong adhesion properties, and high water content, and maintains humid environment, so that the wound is not liable to scab. The gel dressing disclosed by the invention does not contain any irritating ingredients, and is free from toxic and side effects. The gel dressing is used for accelerating wound healing and has a potential application value.
Owner:EAST CHINA NORMAL UNIVERSITY
Pharmaceutical composition and its preparation
InactiveCN103908467AClear ingredientsQuality is easy to controlOrganic active ingredientsPeptide/protein ingredientsFreeze-dryingGuanine nucleoside
The invention belongs to the medicine technical field, and relates to a pharmaceutical composition and a preparation. The pharmaceutical composition comprises amino acid and guanosine hydrate. According to the pharmaceutical composition, the component is clear and the quality is controllable; the pharmaceutical composition can be used for preparing an injection or a freeze-dried powder administration preparation; the pharmaceutical composition has good treatment effect for coronary heart disease and angina pectoris, and is adapted to clinically wide application.
Owner:西藏博睿生物科技有限公司
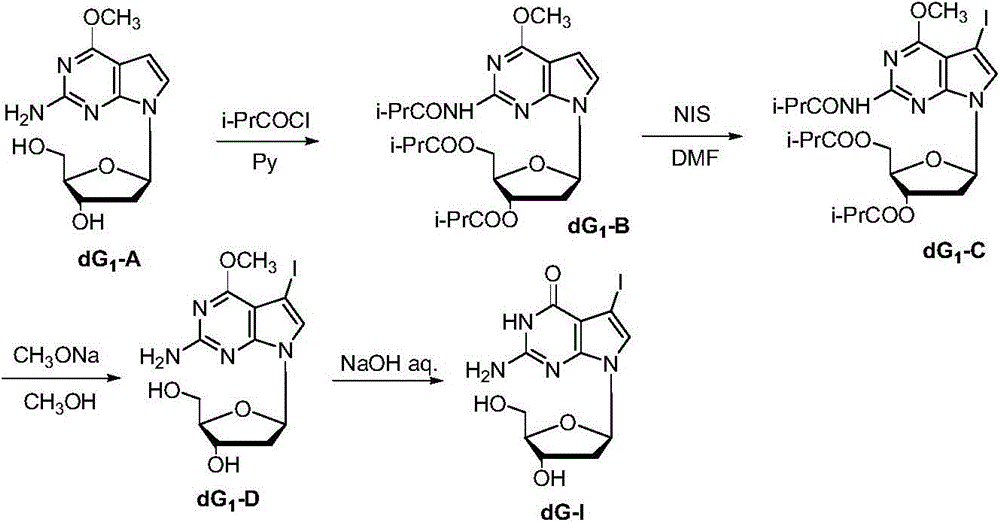
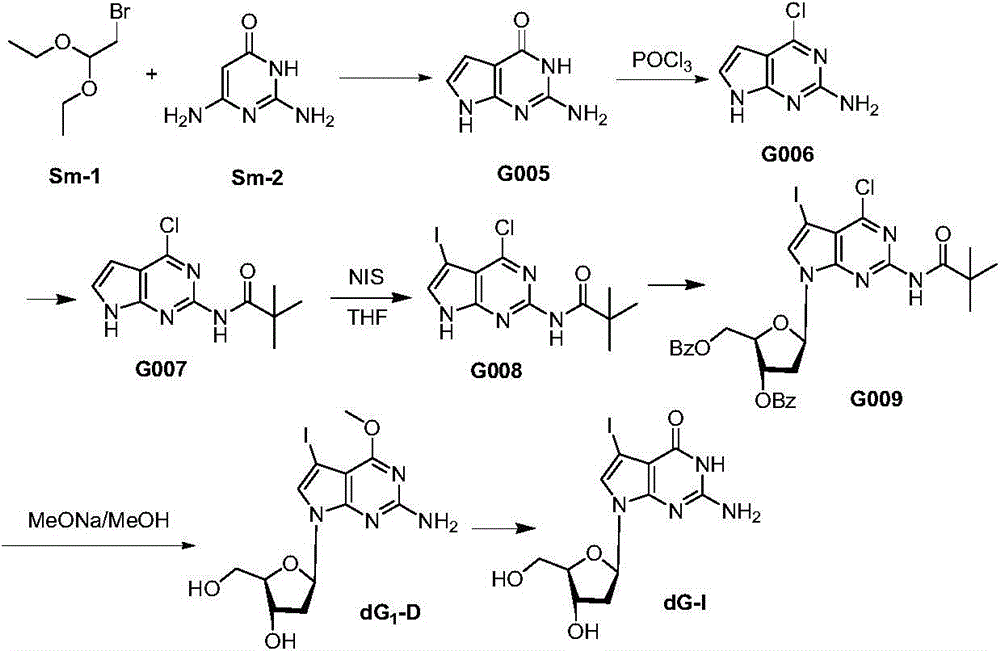
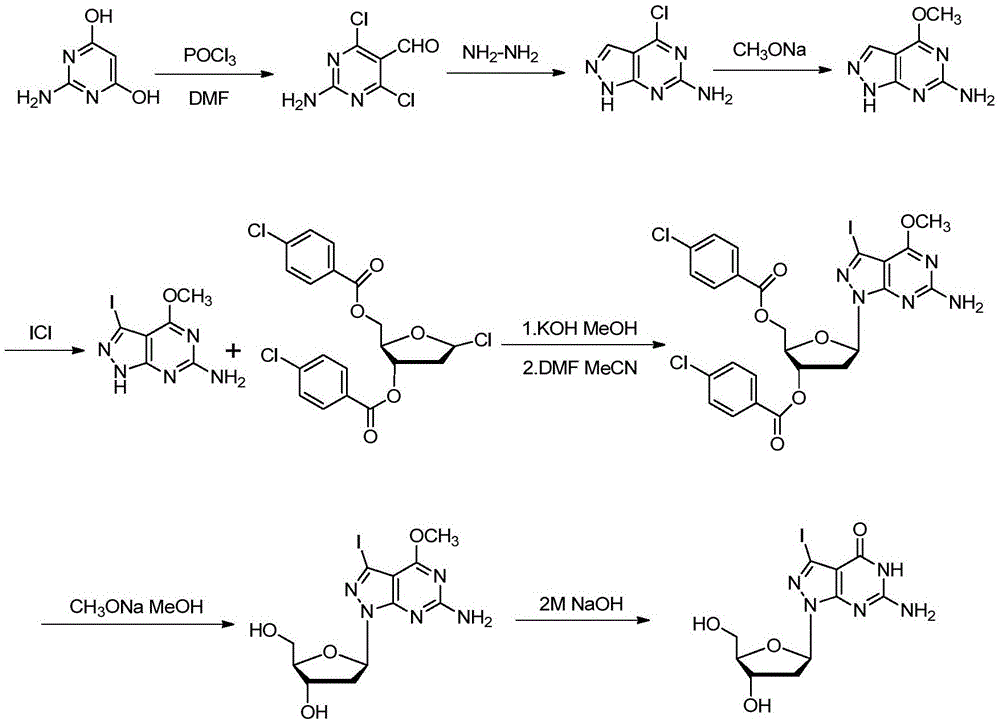
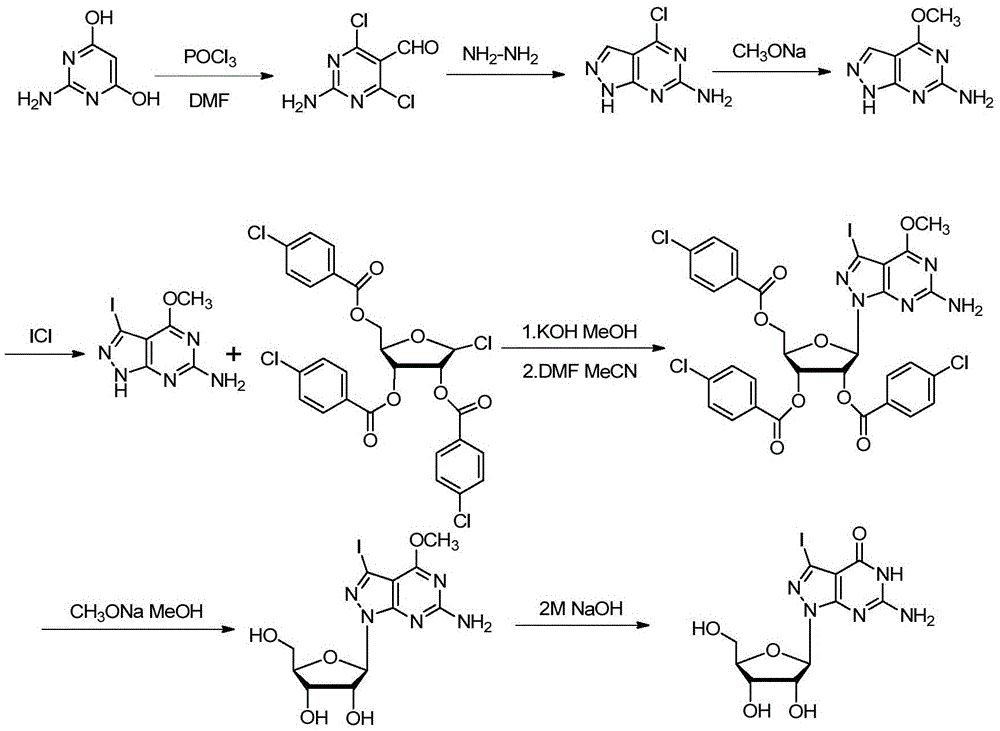
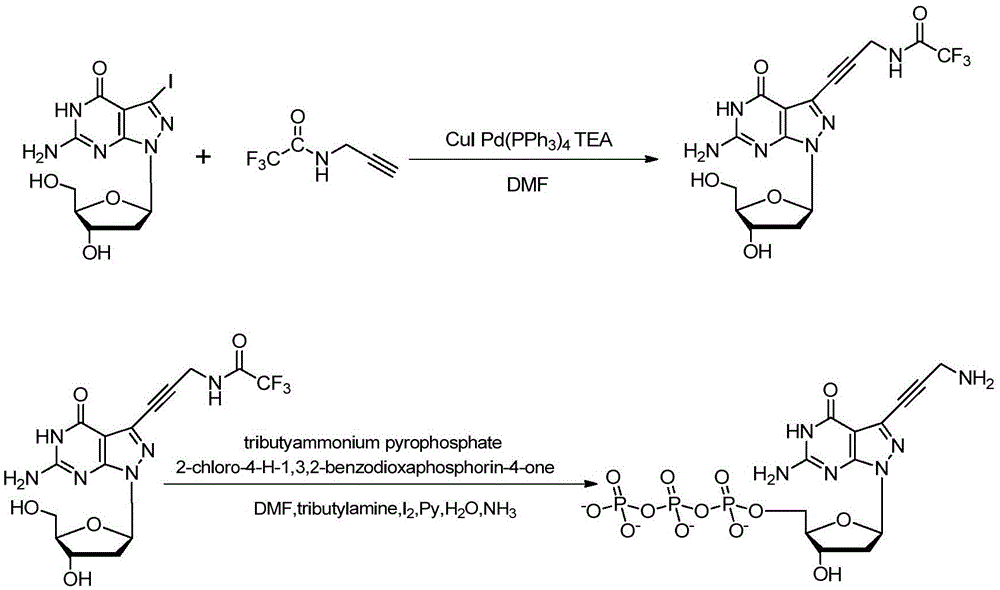
Synthetic method of 7-denitrified-7-substituted guanosine
The invention discloses a synthetic method of 7-denitrified-7-substituted guanosine; the method comprise following steps: a compound with formula (IV 1) or (IV 2) is obtained from a compound with formula (III) by removing protective groups under a alkaline condition; further a compound with formula (I) is obtained by demethylation, that is the 7-denitrified-7-substituted guanosine; wherein, R1 is H or H, R2 is I, Br or Cl, R3 is H or OBz. The synthetic 7-denitrified-7-halogen substituted guanosine is a basic raw material which is widely applied to DNA sequencing, marking, extension and other biological fields, and is very expensive at market with complex synthetic method and difficult control; the synthetic method provided by the invention has the advantages that the raw materials are simple and easily available, and the synthetic process is a routine chemical reaction, and the method can be widely used in a large scale.
Owner:SHANGHAI JIAO TONG UNIV
Method for increasing yield of guanosine produced by fermenting bacillus subtilis
InactiveCN102154415ASynchronous productivity is highImprove conversion rateMicroorganism based processesFermentationGuanine nucleosideNitrogen
The invention discloses a method for increasing yield of guanosine produced by fermenting bacillus subtilis. The method comprises two major technological processes, namely shaking culture of liquid strain and preparation of guanosine fermentation liquor. In the method, preserved strain GDGR-1006 with stable heredity is selected and used; a novel process of adding malic acid and adding glucose liquid and the malic acid in a flowing mode is adopted during fermentation; and thus, the defects of backwardness of the conventional process in the breeding technology, disproportionality of C (Carbon) to N (Nitrogen) of a fermentation medium, low yield of the guanosine from the strain, long fermentation period, low conversion rate and the like are overcome, the strain has high synchronous productivity, high guanosine production level and high guanosine production level by fermentation, the yield of the guanosine can reach over 45g / L, and the conversion rate is over 28 percent.
Owner:XUCHANG RUIDA BIOLOGICAL TECH
New application of guanosine
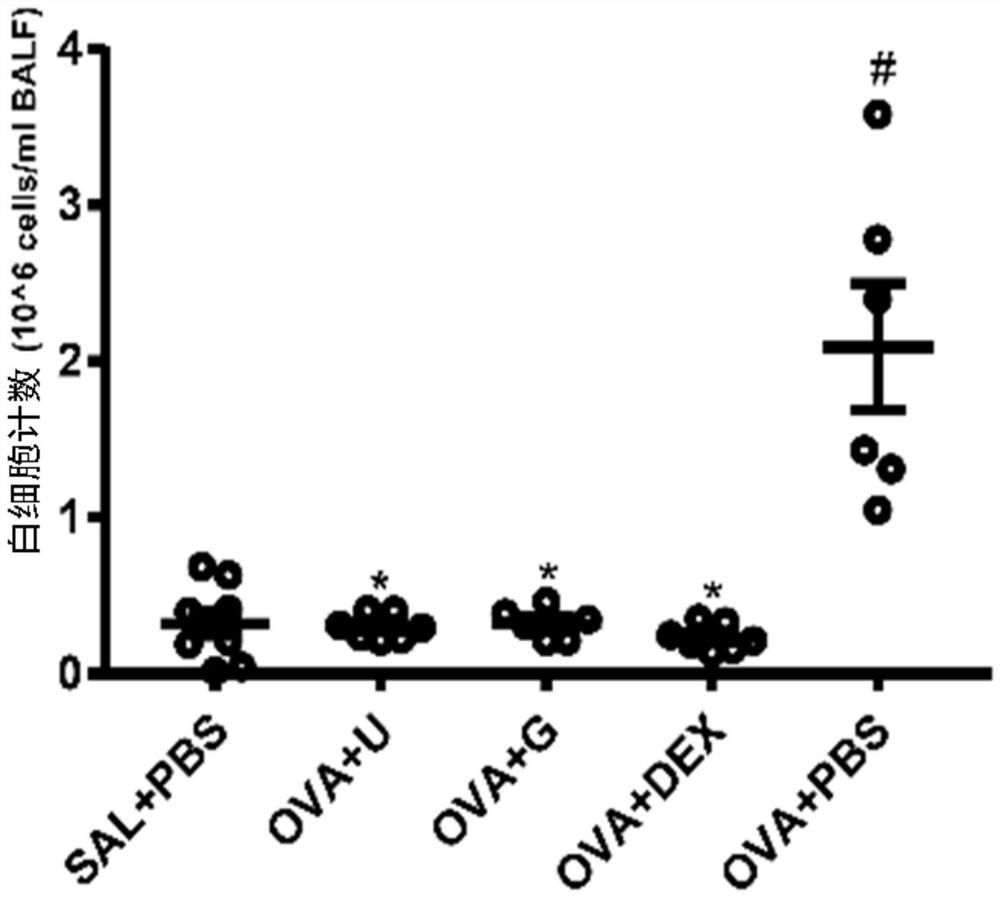
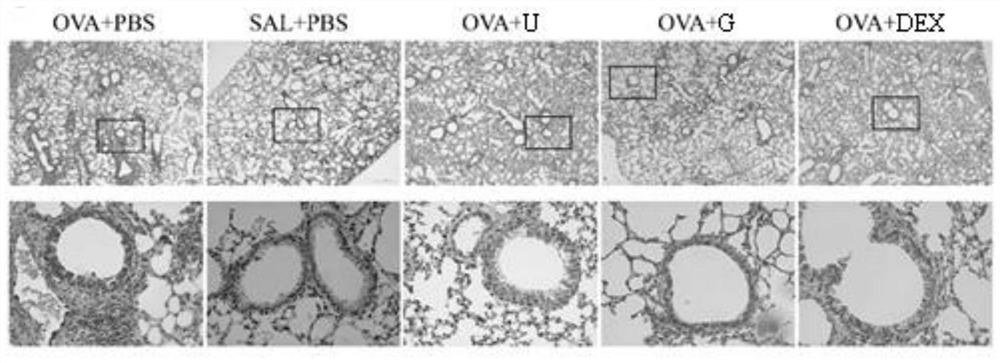
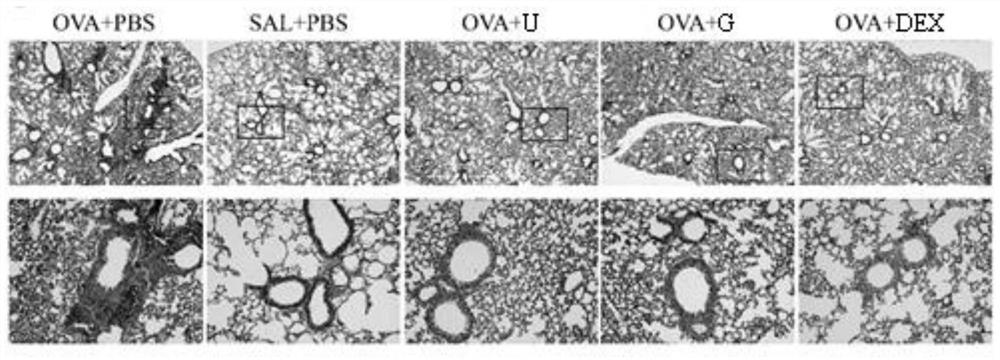
The invention belongs to the technical field of medicines, and particularly relates to new application of guanosine, in particular to new application of guanosine in preparation of medicines for treating asthma or inhibitors of MAPK, NF-kappa B and STAT3. The invention aims to solve the problem that safer and more effective asthma treatment medicines need to be developed urgently in the prior art.It is found that in an in-vitro THP-1-derived macrophage inflammation model, guanosine inhibits generation of a proinflammatory factor IL-6 by inhibiting activation of MAPK and NF-kappa B; in an asthma mouse model, guanosine reduces OVA-IgE of mouse plasma, reduces generation of IL-4, IL-6 and IL-13, relieves airway high reactivity, and relieves lung tissue cell infiltration, airway inflammationand collagen deposition. In addition, the expression levels of p-p38 MAPK, p-p65 NF-kappa B, p-I kappa B alpha and p-STAT3 proteins in lung tissues of mice in a guanosine treatment group are obviouslylower than those in an asthma model group. Therefore, the invention provides application of guanosine in preparation of a p38 MAPK inhibitor, a p65 NF-kappa B inhibitor, an STAT3 inhibitor or an asthma treatment drug.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
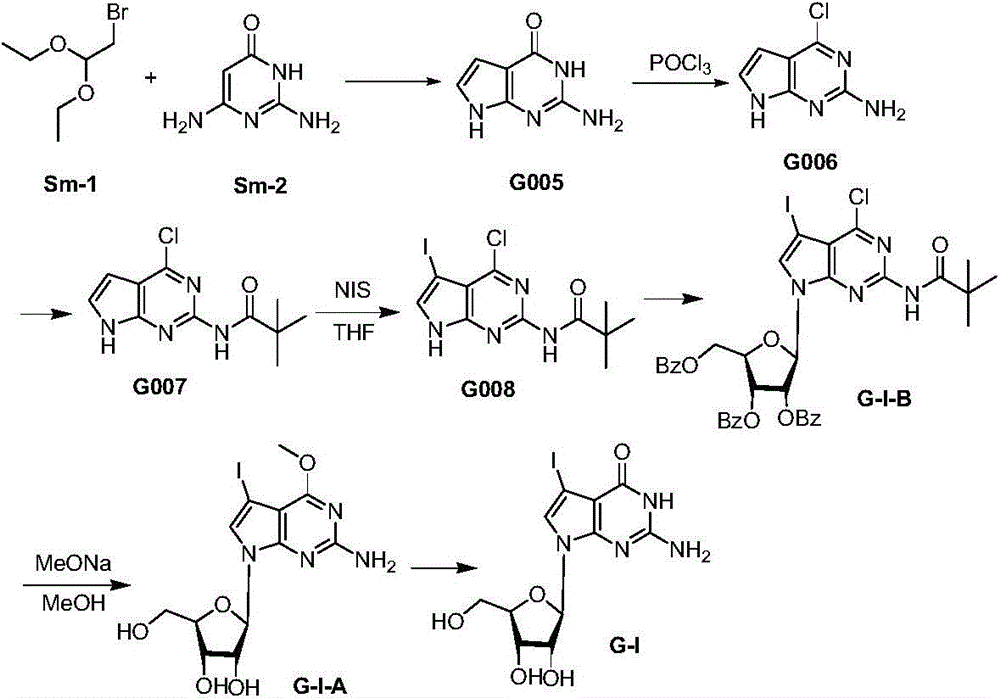
Aza-hybridized guanosine, its synthesis method and its application in dna sequencing
ActiveCN103804447BGood sequencing recognition effectReduce experiment costSugar derivativesMicrobiological testing/measurementChemical synthesisHalogen
The invention discloses hybrid azaguanosine as well as a synthesis method and an application thereof in DNA sequencing. The synthesis method comprises the steps: removing a protecting group of a compound as shown in formula (III) in the specification under an alkaline condition to obtain a compound as shown in formula (II) in the specification; further demethylating to obtain a compound as shown in formula (I) in the specification, i.e. 7-deaza-7-halogen-8-aza-guanosine, wherein R1 is H or OH, R2 is I, Br or Cl, and R3 is H or a compound shown in the specification. The hybrid azaguanosine disclosed by the invention is a novel reagent for DNA sequencing, which, compared to guanosine failing to substitute nitrogen on 8 sites, is more excellent in base identifying effect and more stable in DNA chain structure. Meanwhile, different from the prior art that 8-site nitrogen-substituted guanosine is complex in synthesis method, low in yield and unsuitable for commercial production, the synthesis method disclosed by the invention is easily available in raw material required, and adopts conventional chemical synthesis reaction; and the method is relatively high in yield, and suitable for wide popularization and application.
Owner:SHANGHAI JIAO TONG UNIV
Onion and chicken juice seasoning powder and preparation method thereof
InactiveCN107125690AEnhance secretory functionObvious fragranceYeast food ingredientsXanthosineGARLIC POWDER
The invention discloses onion and chicken juice seasoning powder and a preparation method thereof. The onion and chicken juice seasoning powder consists of the following raw materials in percentage by weight: 45-75% of salt, 9-19% of onion powder, 7-16% of white granulated sugar, 5-10% of garlic powder, 2-7% of fresh chicken powder, 2-7% of black pepper powder, 2-6% of yeast powder, 1-5% of scallion leaves, 1-4% of white pepper powder, 0.5-3% of malt, 0.1-3% of inosine 5'-(trihydrogen diphosphate) disodium salt, 0.1-3% of guanosine 5'-diphosphate disodium salt, 0.1-3% of hot green pepper powder, 0.1-3% of disodium succinate, 0.1-2% of palm oil, 0.1-2% of silicon dioxide, 0.1-1% of vitamin E, 0.1-1% of sodium hexametaphosphate and 0.01-1% of citron yellow. The onion and chicken juice seasoning powder has various distinct delicate fragrance, after an appropriate amount of the onion and chicken juice seasoning powder is added for seasoning during cooking, the fragrance can be obviously enhanced, and the fragrance can perform touch to start the taste function of people; and foods cooked through adding the seasoning powder can significantly increase the secretory function of salivary glands after being put in mouths, and the appetite can be stimulated.
Owner:山东飞达集团有限公司
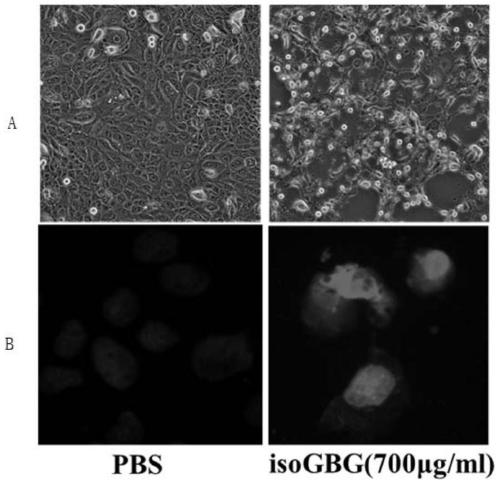
Application of nucleoside hydrogel in preparation of medicine for preventing or delaying canceration of oral mucosa potential malignant diseases
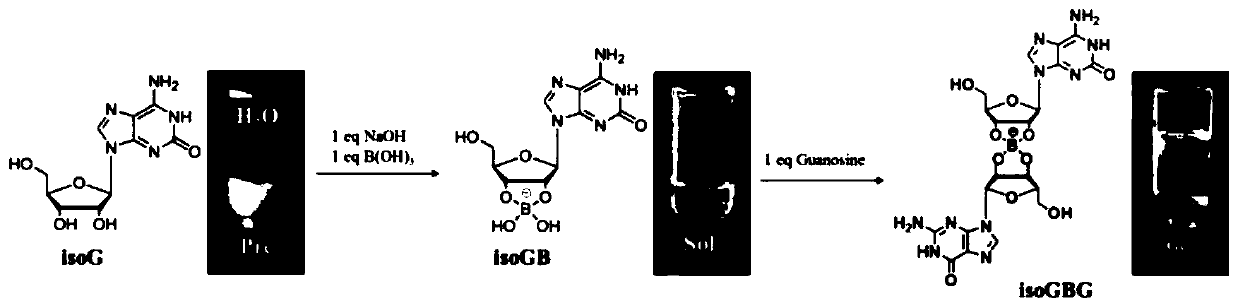
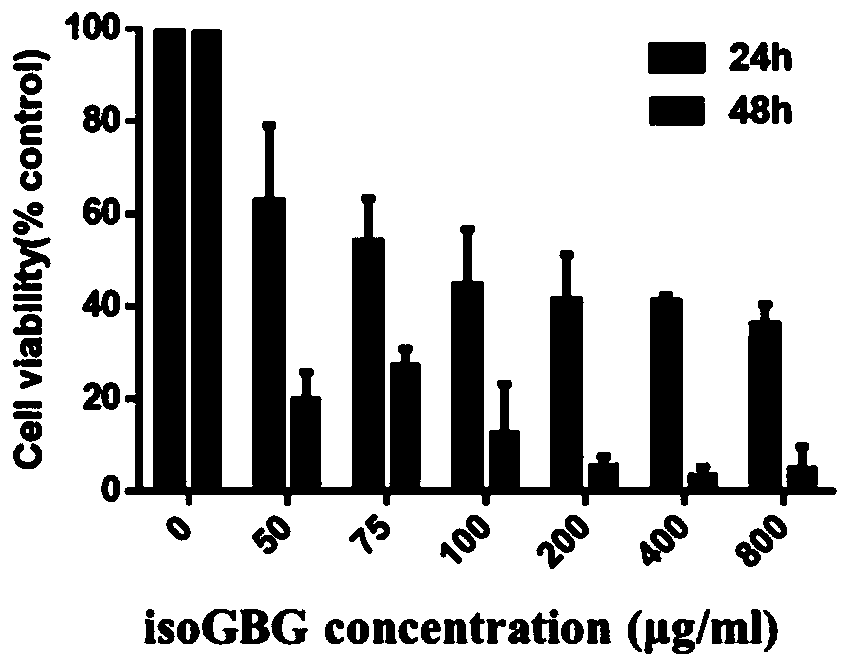
PendingCN111557948APrevent proliferationDelay underlying malignancyOrganic active ingredientsInorganic boron active ingredientsMouth mucosaIsoguanine
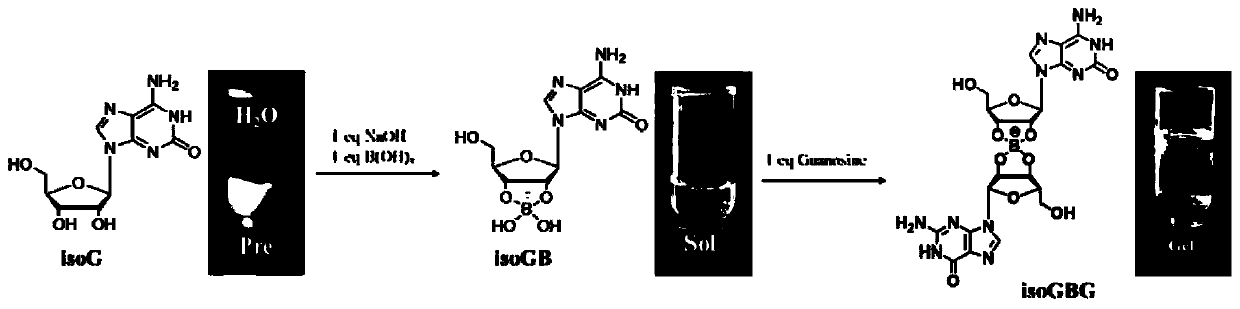
The invention discloses an application of nucleoside hydrogel in preparation of a medicine for preventing or delaying canceration of oral mucosa potential malignant diseases. The nucleoside hydrogel is prepared by dissolving isoguanine nucleoside, guanine nucleoside and borate in water or an aqueous solution and then crosslinking. The experiments prove that the nucleoside hydrogel provided by theinvention can effectively inhibit proliferation of human oral mucosa precancerous lesion cells and delay canceration of oral leukoderma. Therefore, the nucleoside hydrogel provided by the invention has a good application prospect in preparation of drugs for preventing or delaying oral mucosa potential malignant diseases, especially oral leukoplakia canceration.
Owner:SICHUAN UNIV
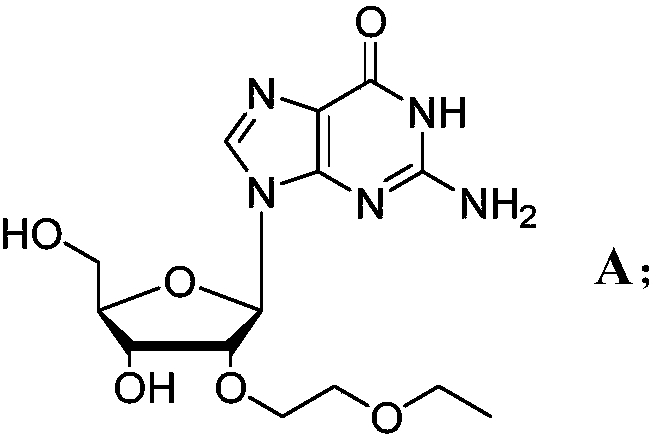
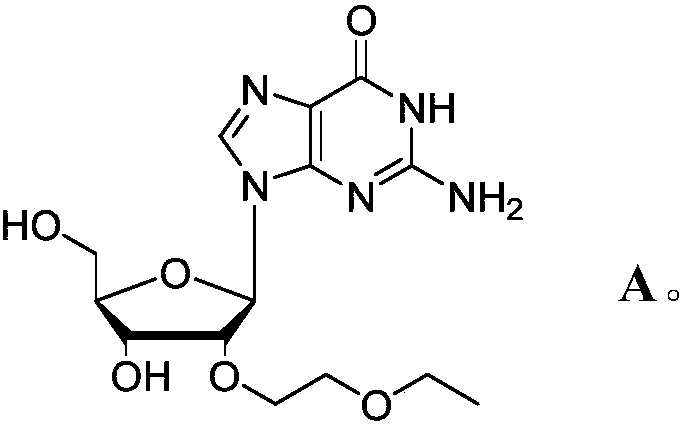
Novel modified nucleoside 2'-EOE-guanosine and preparation method thereof
InactiveCN108822174ASugar derivativesSugar derivatives preparationModified nucleosidesHigh selectivity
The invention relates to a preparation method of a novel modified nucleoside 2'-EOE-guanosine. In the method, 2,6-diamino-guanosine, as an initial raw material, is firstly reacted with a metal hydrideto generate a sodium alkoxide active intermediate, which is then reacted with 2-ethyl halide ethyl ether to generate a 2,6-diamino-guanosine intermediate, modified by a target group; finally througha biochemical reaction, an amino acid, on a specified locus of a compound B, is converted into a carbonyl group, thereby generating the target product, 2'-EOE-guanosine, at high yield and high selectivity. The method can easily and economically mass-synthesize the 2'-EOE-guanosine.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
Method for preparing acyclovir
InactiveCN108822108AFeeding is simpleSimple post-processingOrganic chemistryAcetic anhydrideGuanine nucleoside
The invention discloses a method for preparing acyclovir, comprising synthesis of diacetylguanine, namely guanosine, acetic anhydride and boric acid are added to a reaction kettle at a weight ratio of1:6.4:0.006, the temperature is increased to 110-120 DEG C, and heat preservation is conducted for 6-12 hours; then the temperature is reduced to 60-100 DEG C, and heat preservation is conducted for6 hours, so the reaction is finished; subsequently, a part of the reaction liquid is distilled off under reduced pressure, the temperature is lowered to 3-5 DEG C to be maintained for 5 hours, discharging and centrifugal separation are conducted, rinsing with acetic anhydride is conducted for 3 times, and drying is conducted to obtain diacetylguanine. The method for preparing acyclovir provided bythe invention has the advantages that the process is easy to feed, the post-treatment is simple, the cost of the three-waste treatment is reduced, and ethanol is used as a recrystallization solvent,which not only reduces the cost, but also improves the product quality.
Owner:ZHEJIANG ZHEBEI PHARM CO LTD
Biomolecule with therapeutic tumour action and its use
InactiveCN1528776AOvercome toxic side effectsInhibition of telomerase activityOrganic active ingredientsSugar derivativesSide effectUracil nucleoside
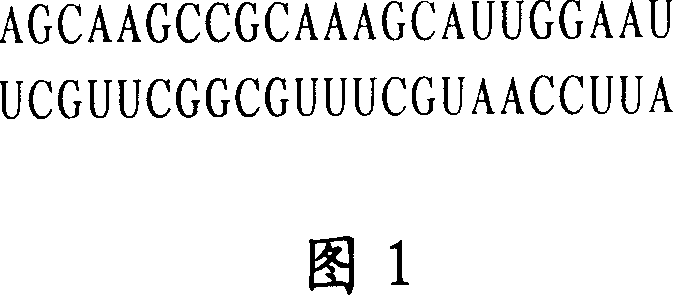
The invention is a biomolecule curing tumours, its character: in a special sequence, it is a dichain RNA molecule with 23 basic groups. Its molecular structure contains adenine nucleotide A, guanosine G, cytidine C and uridine U. Its beneficial effects: 1, obvious effect of prohibiting tumour growth and high selectivity, able to overcome poisonous side effect of quinoline drug; 2, good stability; 3, effect amplification; 4, easy to prepare. It can be used to prepare clinical antitumor drug, where the drug form is any one of water solution injection, liposome soliquoid injection and latex.
Owner:TIANJIN SAIER BIOTECH
Inhibitory oligonucleotides and their use in therapy
Inhibitory oligonucleotide having the general formula:X1 C C N1 N2 N3 X2 N4 N5 GGG N6 X3 N7 (I)are disclosed which can be used in pharmaceutical compositions, whereby in formula (I)C is cytidine or a derivative thereof, whereby the cytidine derivative is selected from the group consisting of 5-methylcytidine, a cytidine-like nucleotide having a chemical modification involving the cytosine base, cytidine nucleoside sugar, or both the cytosine base and the cytidine nucleoside sugar, 2′-O-methylcytidine, 5-bromocytidine, 5-hydroxycytidine, ribocytidine and cytosine-β-D-arabinofuranoside,G is guanosine or a derivative thereof, whereby the guanosine derivative is selected from the group consisting of 7-deazaguanosine, a guanosine-like nucleotide having a chemical modification involving the guanine base, the guanosine nucleoside sugar or both the guanine base and the guanosine nucleoside sugar,X1 and X3 is any nucleotide sequence with 0 to 12 bases and each nucleotide is independent of any other, X2 is any nucleotide sequence having 0 to 3 nucleotides,N1, N2 and N3are each independently any nucleotide,N4 and N7 is a pyrimidine or a modified pyrimidine,N5 is a purin or a modified purin,N6 is a modified pyrimidine, A or a modified purin,wherein at least two of the nucleotides N4, N5, N6 or N7 are modified purins or modified pyrimidines.
Owner:SAREPTA THERAPEUTICS INC
Strontium ion responsive high polymer material, ion imprinted gel, gel grating, preparation methods of ion imprinting gel and gel grating, and strontium ion detection method
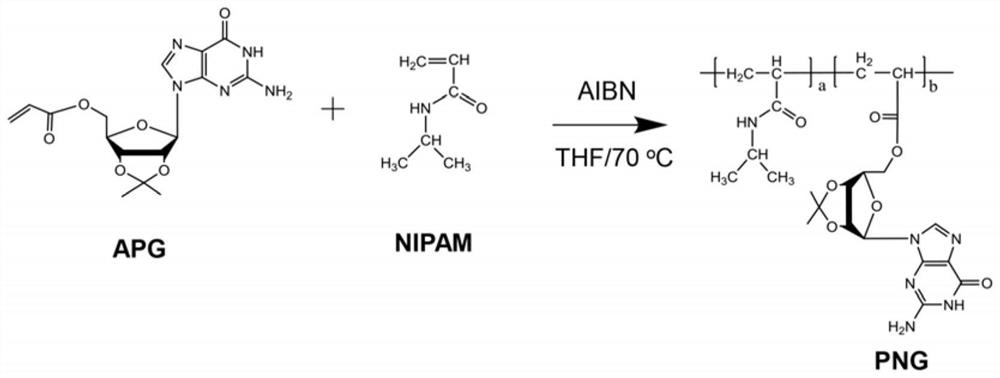
ActiveCN113174008AExcellent temperature sensitive performanceReduce light transmittanceScattering properties measurementsAcyl groupPolymer chemistry
The invention provides a Sr<2+> responsive high polymer material, an ion imprinted gel and a gel grating. The high polymer material is formed by copolymerization of N-isopropylacrylamide and 5'-O-acryloyl-2', 3'-O-isopropylidene-guanosine, and the ion imprinted gel is formed by A G-tetramer unit, formed by copolymerization of 5'-O-acryloyl-2 ' 3'-O-isopropylidene-guanosine under induction of Sr<2+>, and Sr<2+>-containing gel formed by copolymerization crosslinking of N-isopropylacrylamide after Sr<2+> in a gel network is fully eluted, and the gel grating is composed of a substrate and parallel gel strips on the substrate. Each gel strip is composed of the ion imprinted gel. The invention also provides a trace Sr<2+> detection method. The environmental protection property of the Sr<2+> detection material can be improved, the detection difficulty of the Sr<2+> detection material is reduced, and sensitive and convenient detection of trace Sr<2+> is realized.
Owner:SICHUAN UNIV
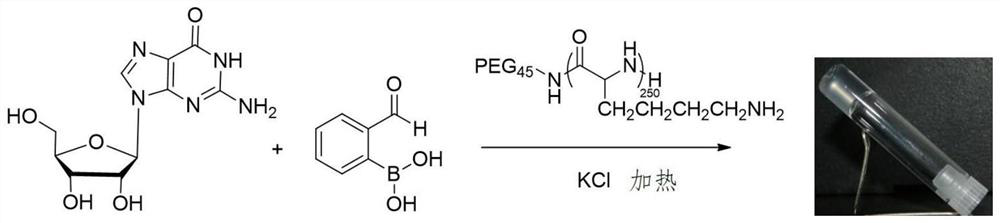
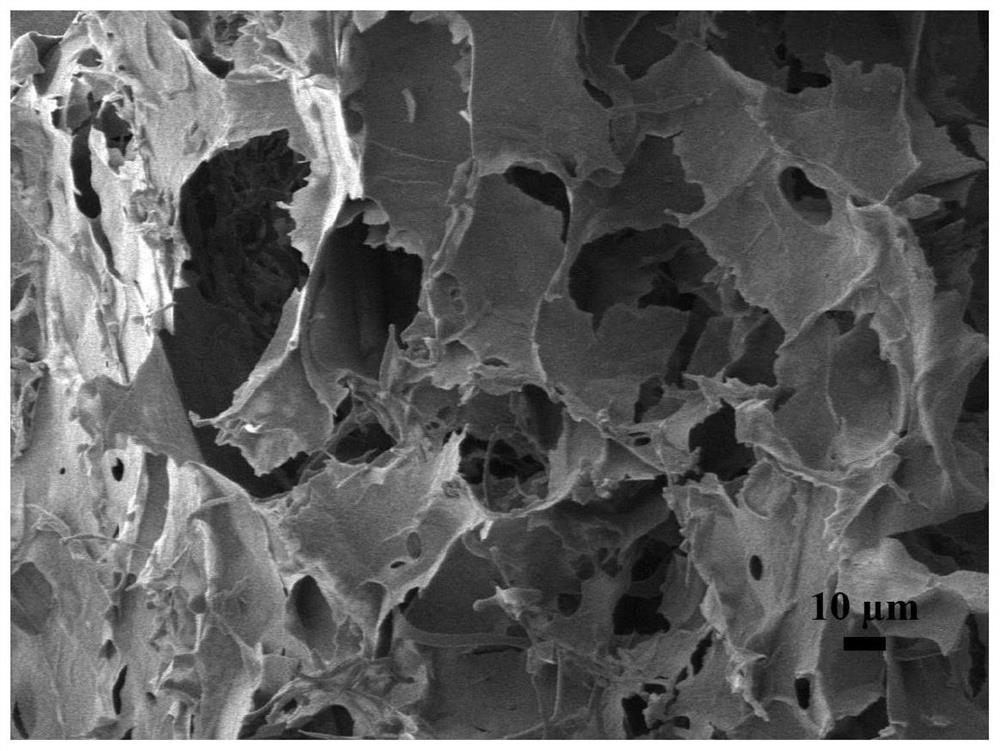
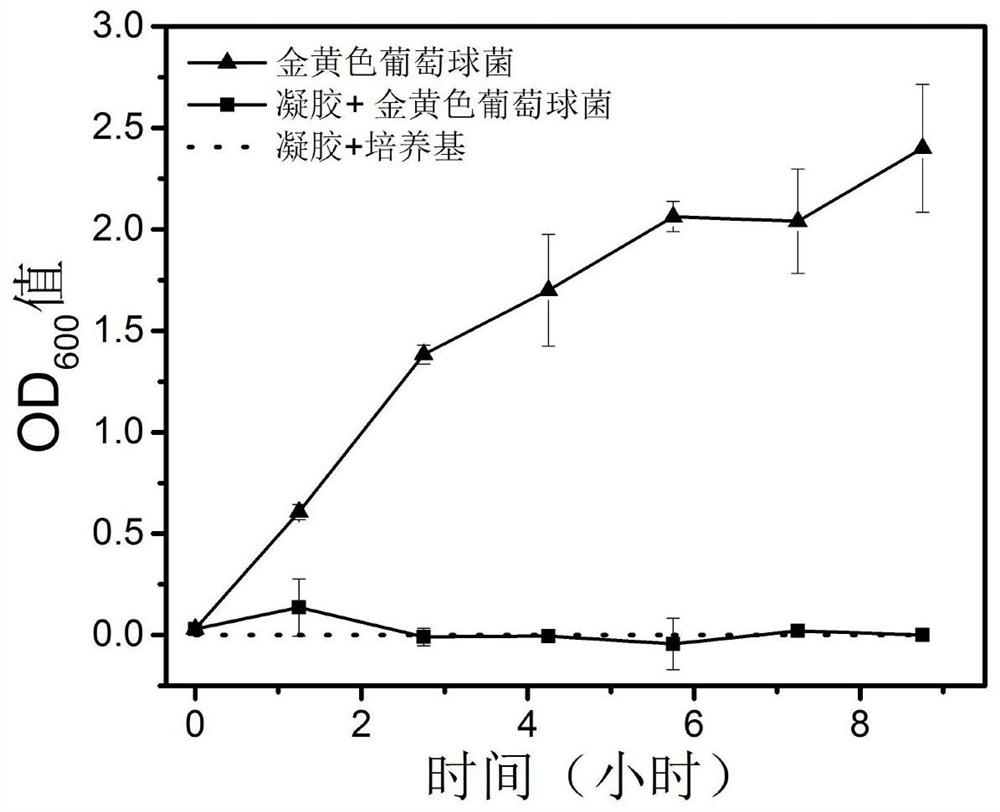
Preparation method of hydrogel with g-quadruplex structure and its application in killing Staphylococcus aureus and Escherichia coli
ActiveCN107333755BLow biological toxicityHigh yieldBiocideOrganic chemistryPolyethylene glycolBoronic acid
The invention relates to a preparation method of a hydrogel having a G-quadruplex structure, and applications of the hydrogel in killing of Staphylococcus aureus and Escherichia coli, wherein a G-quadruplex structure is formed by using guanosine as a main body, and polyethylene glycol-b-polylysine having antibacterial performance is introduced to form the G-quadruplex hydrogel. According to the preparation method, guanosine forms a G-quartet under the stabilization of potassium ions, the vicinal diol of the guanosine and 2-formylbenzeneboronic acid form a dynamic boron ester bond, the primary amine in the polyethylene glycol-b-polylysine having a broad-spectrum antibacterial performance and the aldehyde group of the 2-formylbenzeneboronic acid form a dynamic imine bond, and the G-quartets are connected to form the G-quadruplex through the formations of the boron ester bond and the imine bond so as to form the antibacterial hydrogel. According to the present invention, the preparation method has advantages of simple and easily available raw materials, simple synthesis step, high yield and batch production; and the obtained hydrogel has advantages of stability, low biological toxicity, broad-spectrum antibacterial effect, efficient Gram positive bacterial / Gram negative bacterial killing, and easy promotion and application.
Owner:NANKAI UNIV
Biomolecule with therapeutic tumour action and its use
InactiveCN1237070COvercome toxic side effectsInhibition of telomerase activityOrganic active ingredientsSugar derivativesSide effectUracil nucleoside
The invention is a biomolecule curing tumours, its character: in a special sequence, it is a dichain RNA molecule with 23 basic groups. Its molecular structure contains adenine nucleotide A, guanosine G, cytidine C and uridine U. Its beneficial effects: 1, obvious effect of prohibiting tumour growth and high selectivity, able to overcome poisonous side effect of quinoline drug; 2, good stability; 3, effect amplification; 4, easy to prepare. It can be used to prepare clinical antitumor drug, where the drug form is any one of water solution injection, liposome soliquoid injection and latex.
Owner:TIANJIN SAIER BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com