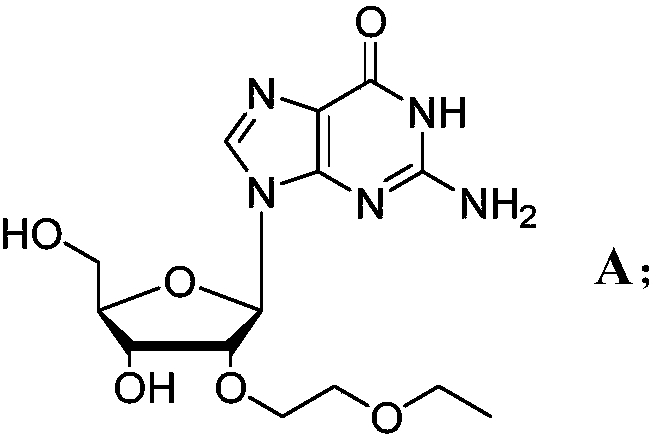
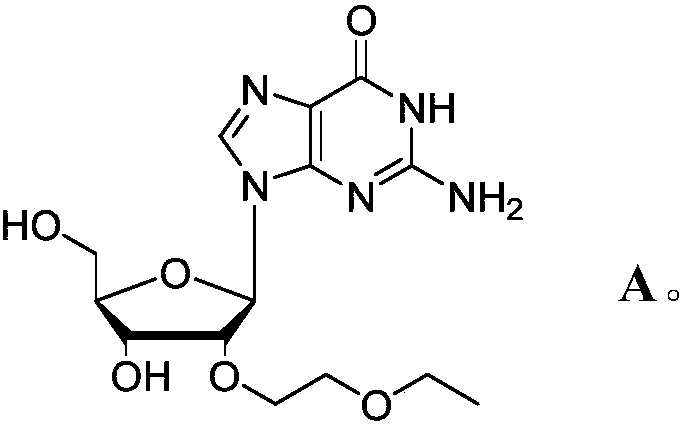
Novel modified nucleoside 2'-EOE-guanosine and preparation method thereof
A purine nucleoside intermediate and compound technology, applied in the field of nucleoside compound synthesis, can solve problems such as inability to meet industrialization requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
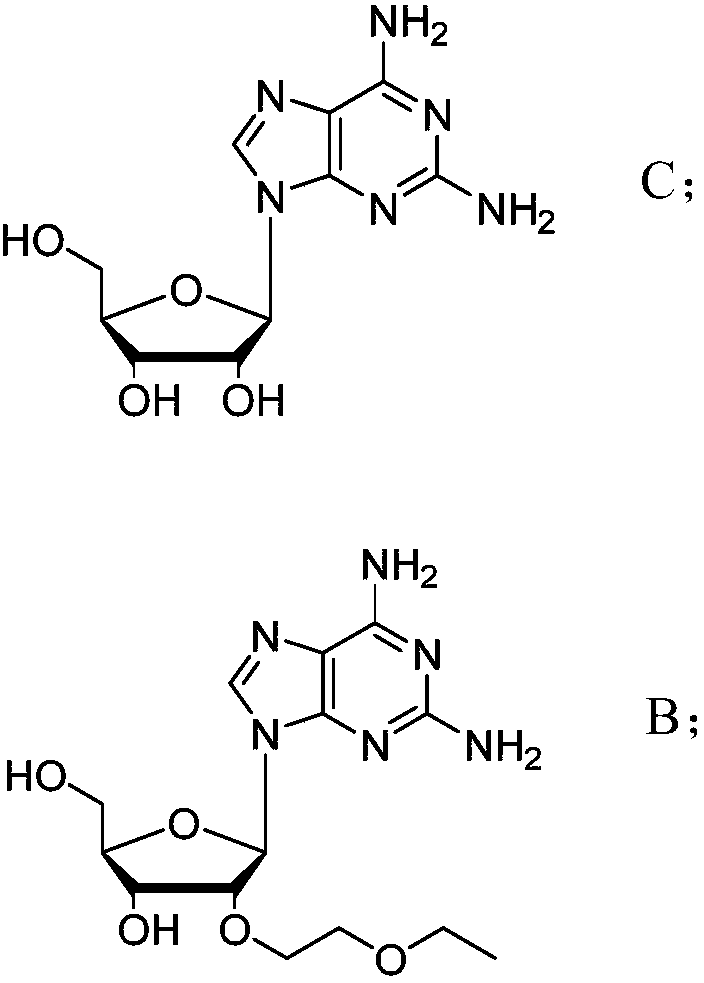
[0032] The preparation method of the compound of formula B comprises: using compound C (2,6-diamino-purine nucleoside) as a starting material, first reacting with a metal hydride to generate a sodium alkoxide active intermediate; then reacting with 2- Haloethyl ethyl ether reaction. Its synthetic route is as follows:
[0033]
[0034] After obtaining the above intermediate, further, the present invention provides a method suitable for industrial production to prepare 2'-EOE-guanosine (compound of formula A), comprising: under neutral conditions, preferably pH=7 Under -8 conditions, the 2,6-diamino-purine nucleoside intermediate B is catalyzed by deaminase, and the amino group at a specific site is converted into a carbonyl group, thereby obtaining compound A (2'-EOE-guanine nucleoside) . Its synthetic route is as follows:
[0035]
[0036] In the synthesis of each step involved in the present invention, Solvent 1 can select DMF, DMSO or DME. It should be understood t...
Embodiment 1
[0068] The preparation of embodiment 1,2'-ethoxyethyl (EOE)-2,6-diaminopurine nucleoside (compound B)
[0069]
[0070] Weigh 135.58kg of 2,6-diaminopurine nucleoside (CAS No.: 2096-10-8) and dissolve it in 1350L DMF, cool the reaction solution to 0±2°C, add 29.10kg of NaH under the protection of argon, and add the process temperature 0°C to 5°C, stir at 0°C for 1h after addition.
[0071] After the above reaction system naturally returned to room temperature, 73.64 kg of 2-bromoethyl ethyl ether was added dropwise to the system, and the reaction was stirred at room temperature after the dropwise completion. React until the product no longer increases. Slowly add water to the system at 0±2°C to quench the reaction. Slowly add 2M HCl solution to the system to adjust the pH to neutral. Concentrate in vacuo at 65°C until it does not drop, and the residual solid is crystallized in ethanol to obtain 72.91kg of light yellow solid, which is 2'-EOE-2,6-diaminopurine nucleoside. ...
Embodiment 2、2
[0073] The preparation of embodiment 2,2'-EOE-guanosine (compound A)
[0074]
[0075] Weigh 52.91kg of the 2'-EOE-2,6-diaminopurine nucleoside obtained in Example 1 above, add 1060L of water, add deaminase after the pH test paper detection system is neutral, and stir at 37-42°C for reaction. After the reaction, the reaction solution was concentrated to a small volume in an oil pump water bath at 55±2°C, and a large amount of white crystals were precipitated, then the system was kept in an ice bath at 0°C for 1 hour, filtered, the filter cake was washed with ice water, recrystallized once with water, and centrifuged After drying, 47.64 kg of the product was obtained, which was 2'-EOE-guanosine. Purity: 99.6%, yield: 90.0%.
[0076] 1 H NMR (600MHz, DMSO-d 6 )δ (ppm): 10.63 (s, 1H), 7.95 (s, 1H), 6.46 (brs, 2H), 5.78 (d, J = 6.6Hz, 1H), 5.06 (t, J = 5.4Hz, 1H) ,5.04(d,J=4.8Hz,1H), 4.38-4.36(m,1H),4.26-4.23(m,1H),3.91-3.89(m,1H),3.68-3.64(m,1H), 3.61 -3.50(m,3H),3.44-3.4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com