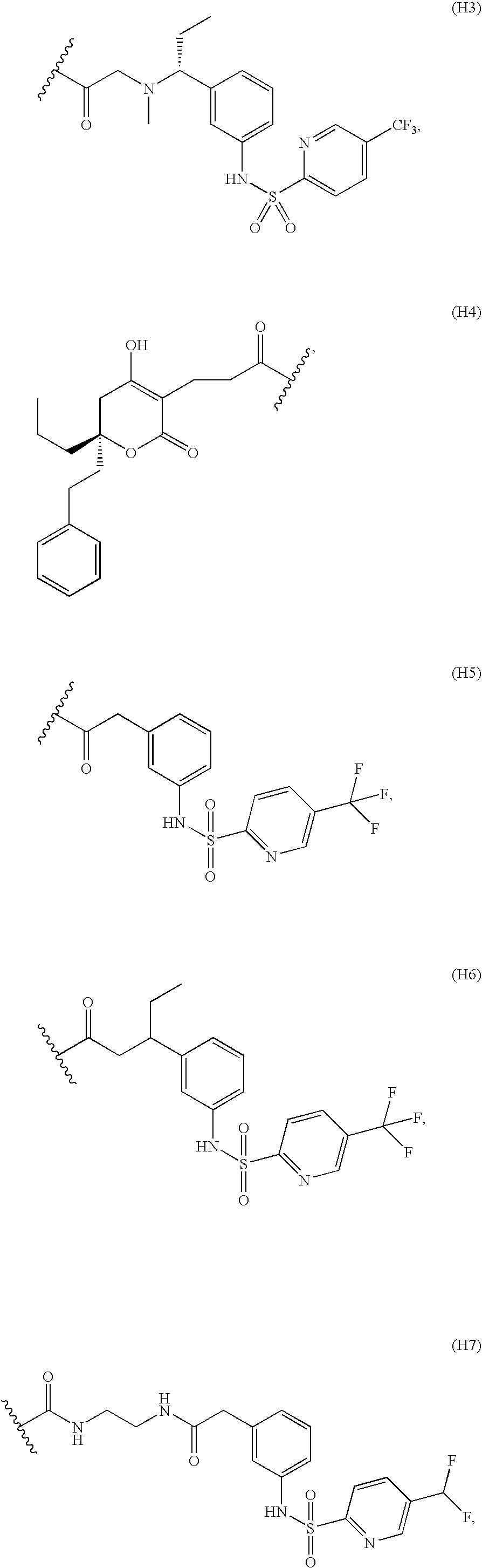
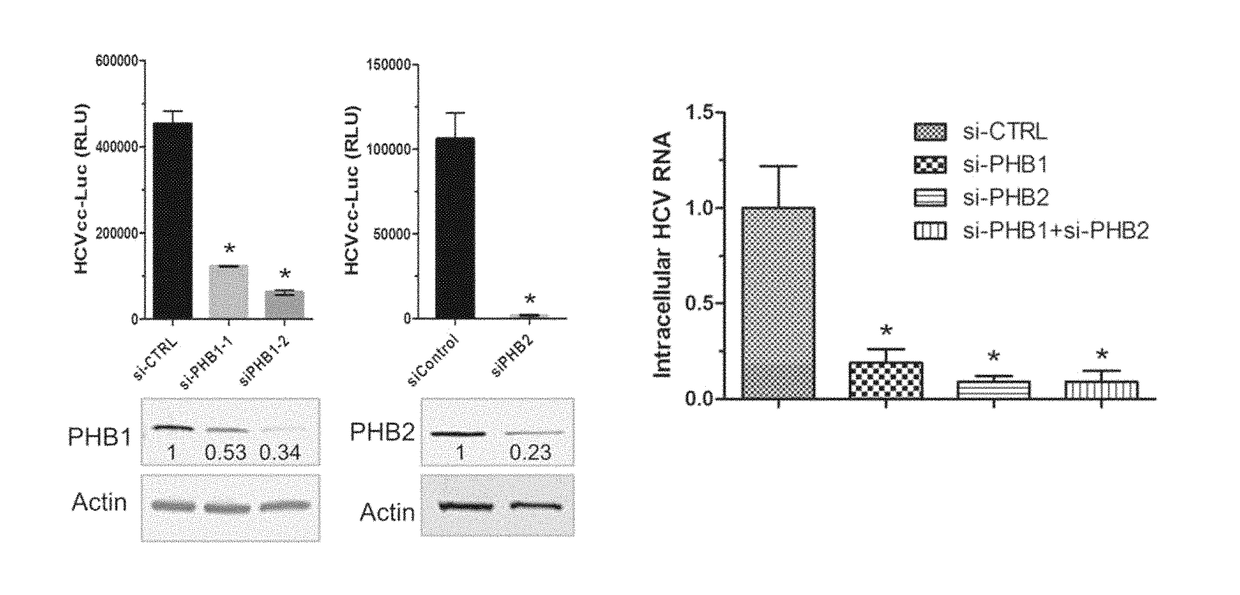
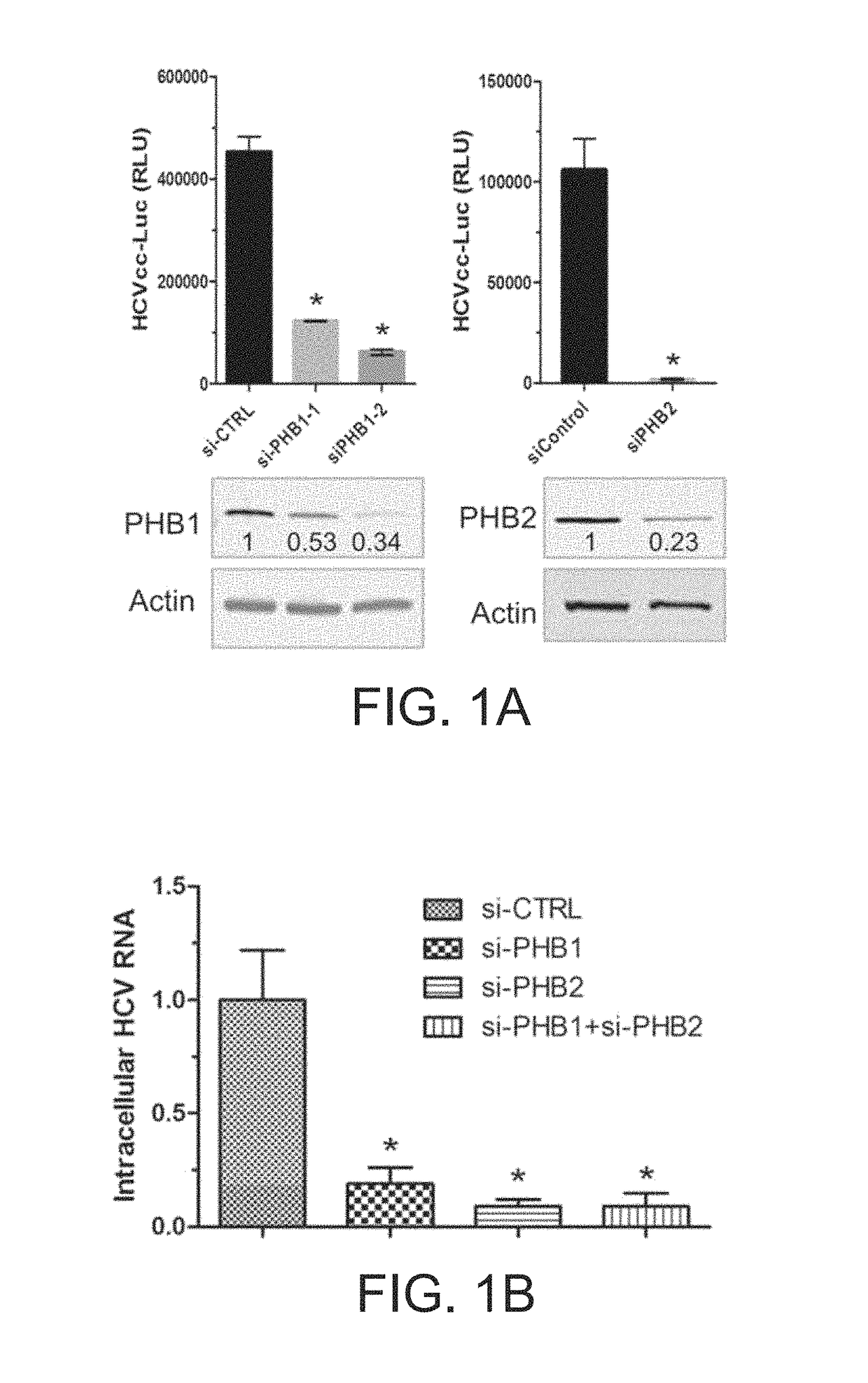
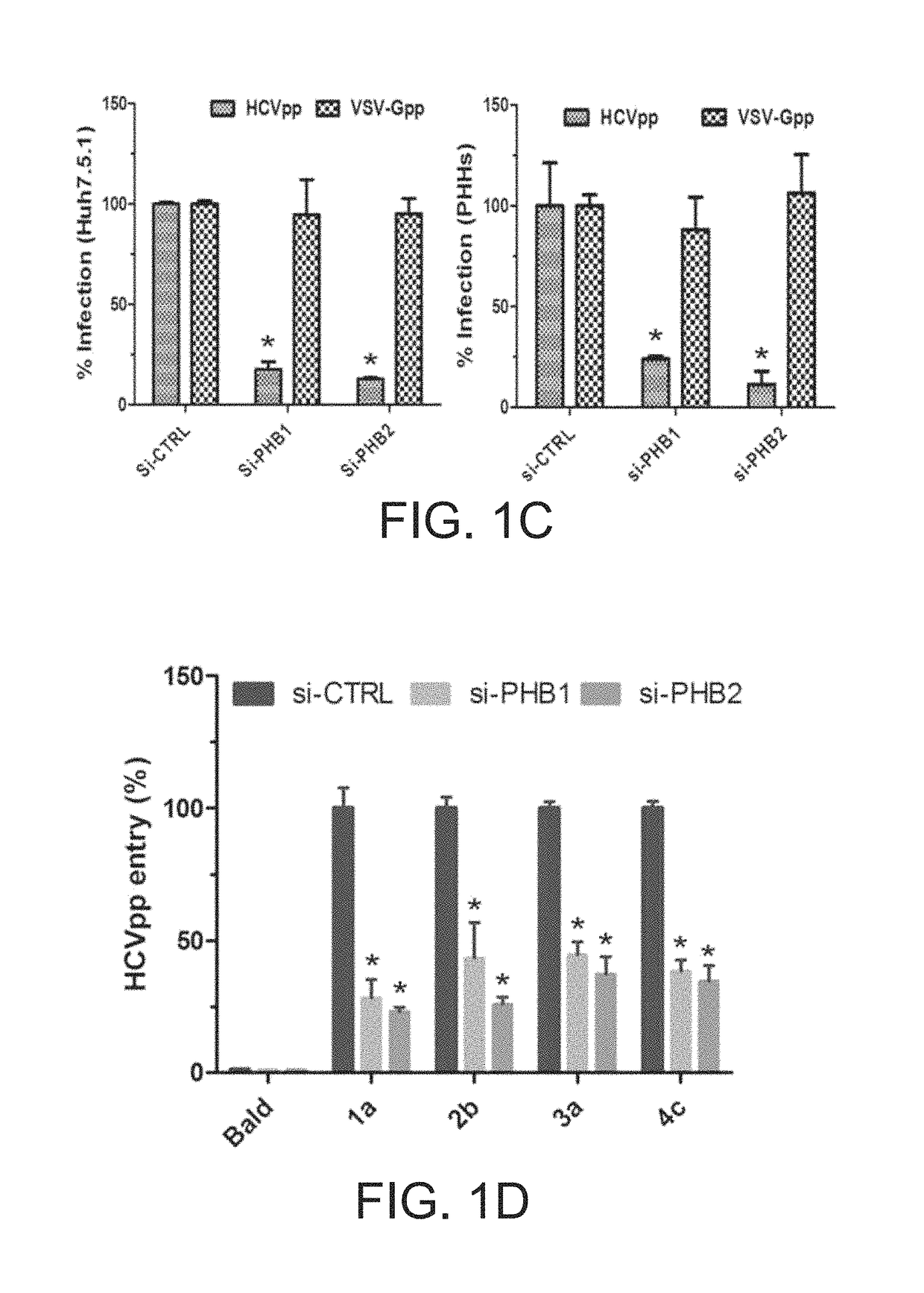
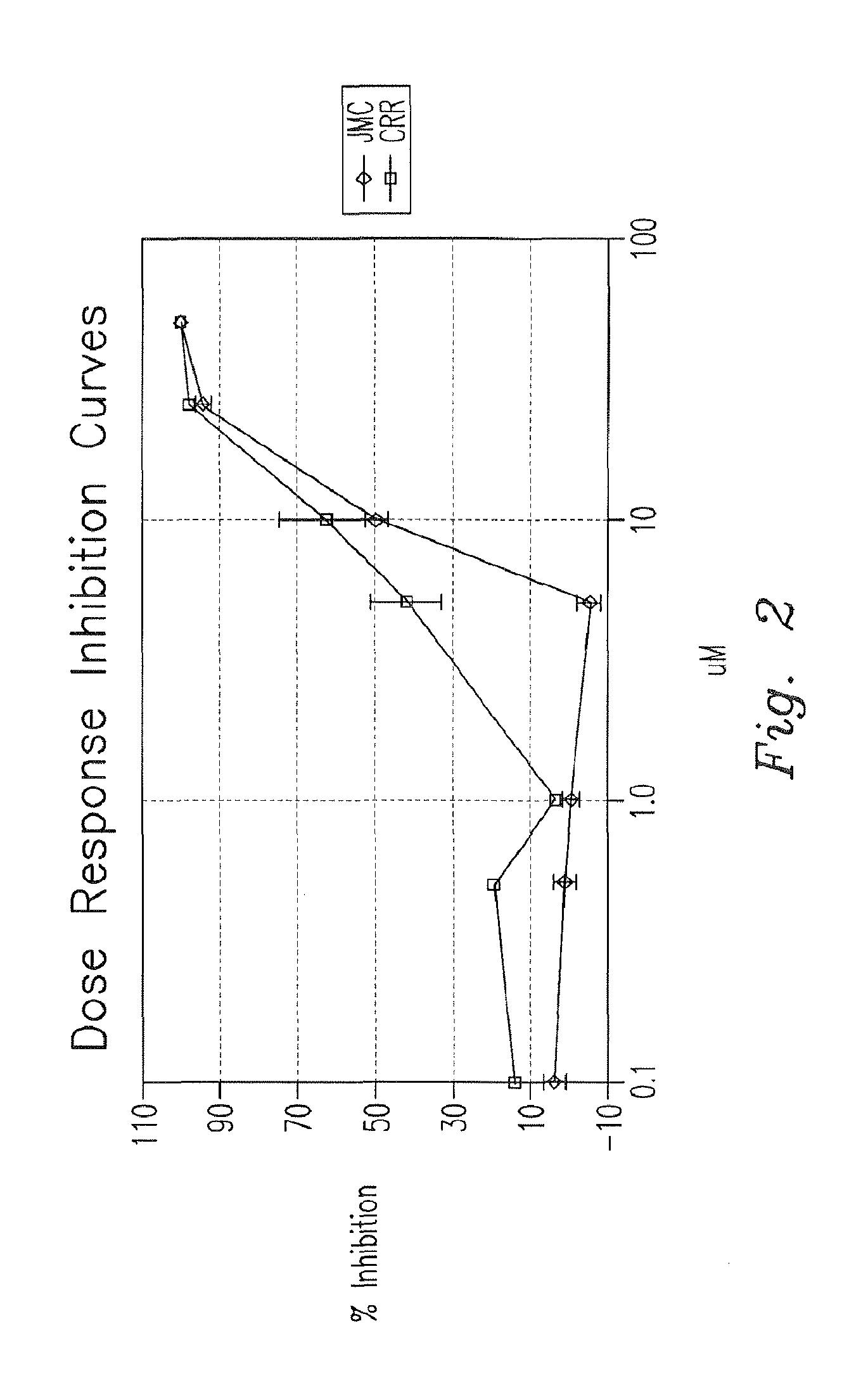
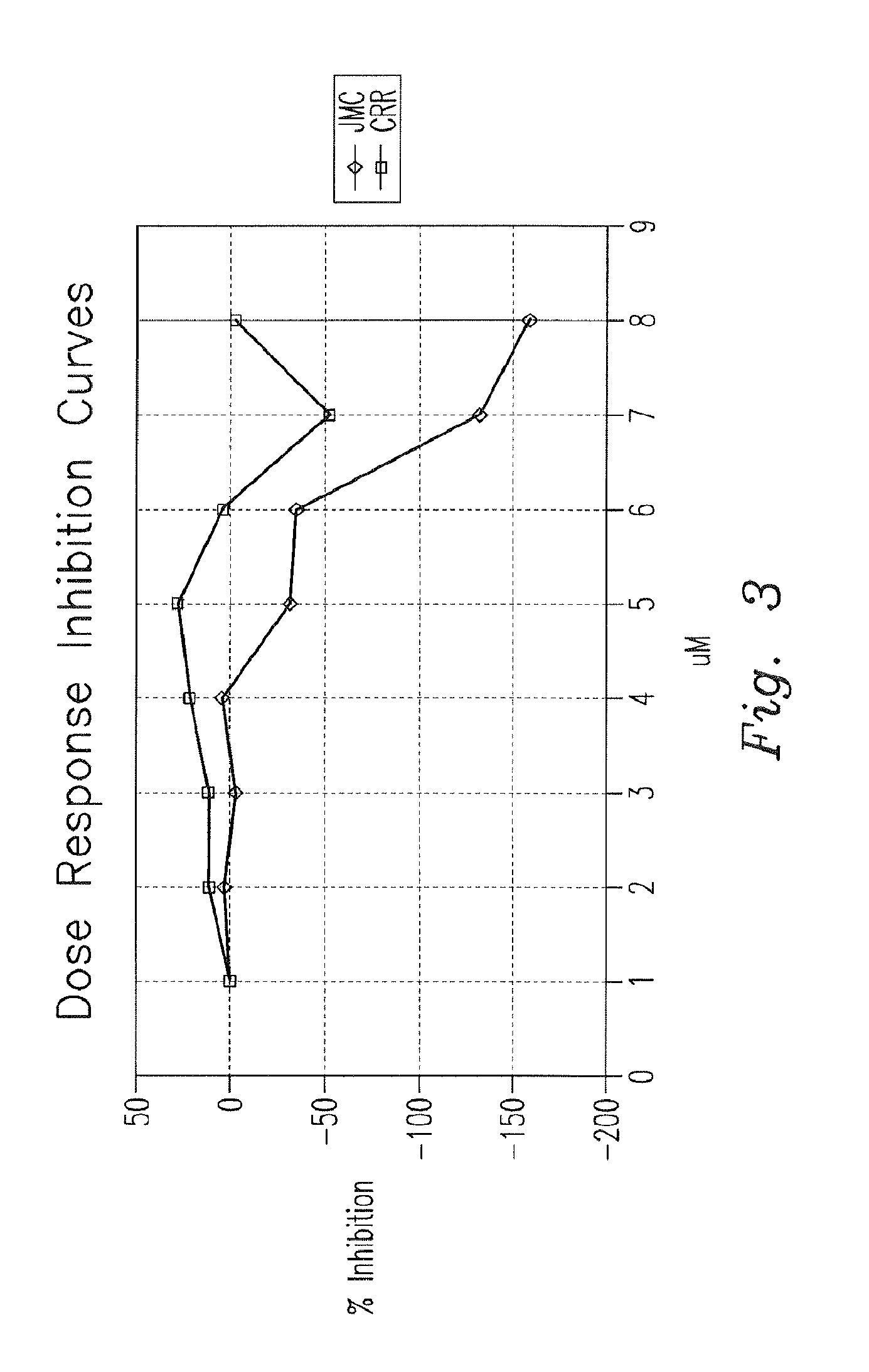
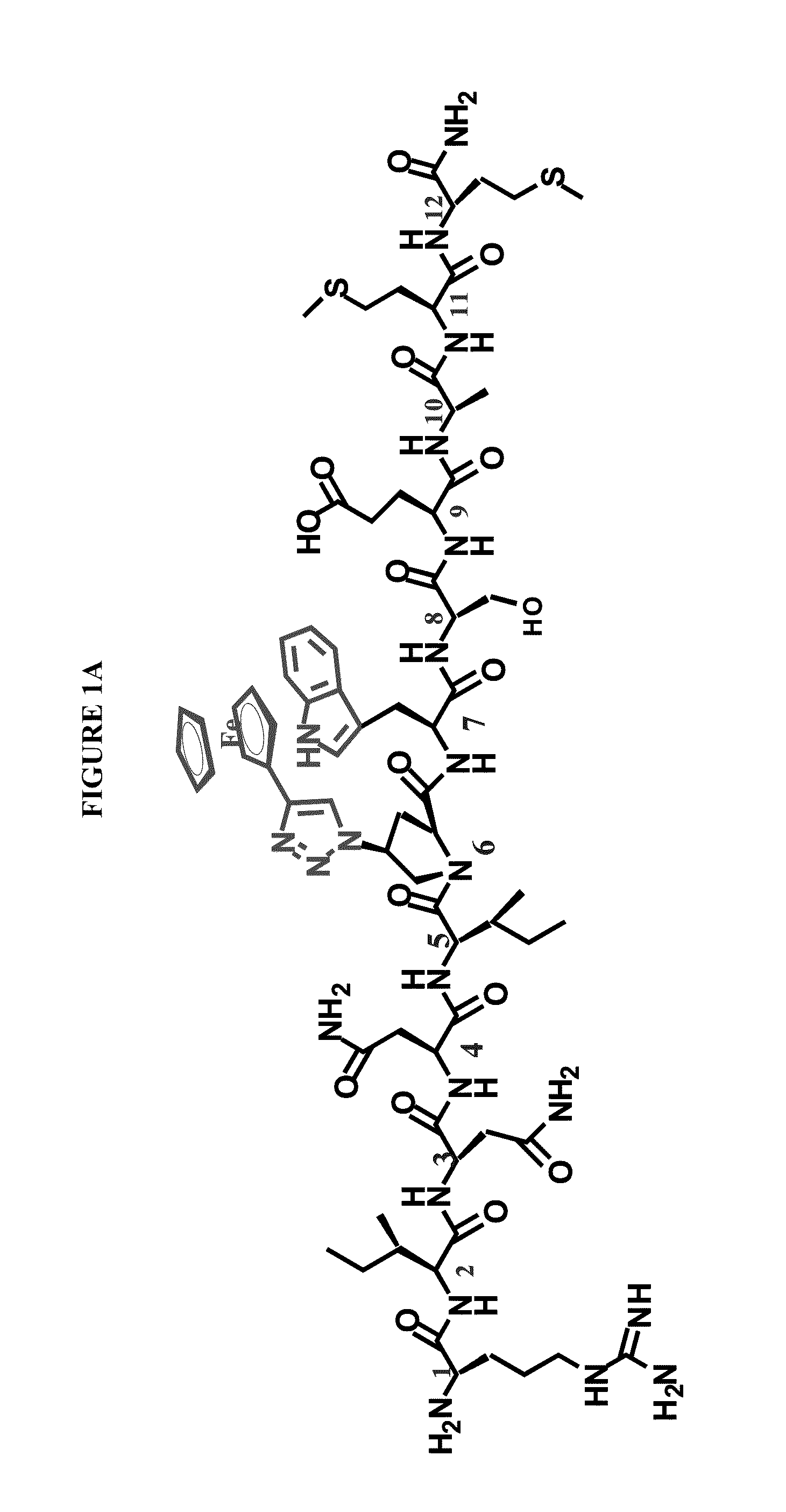
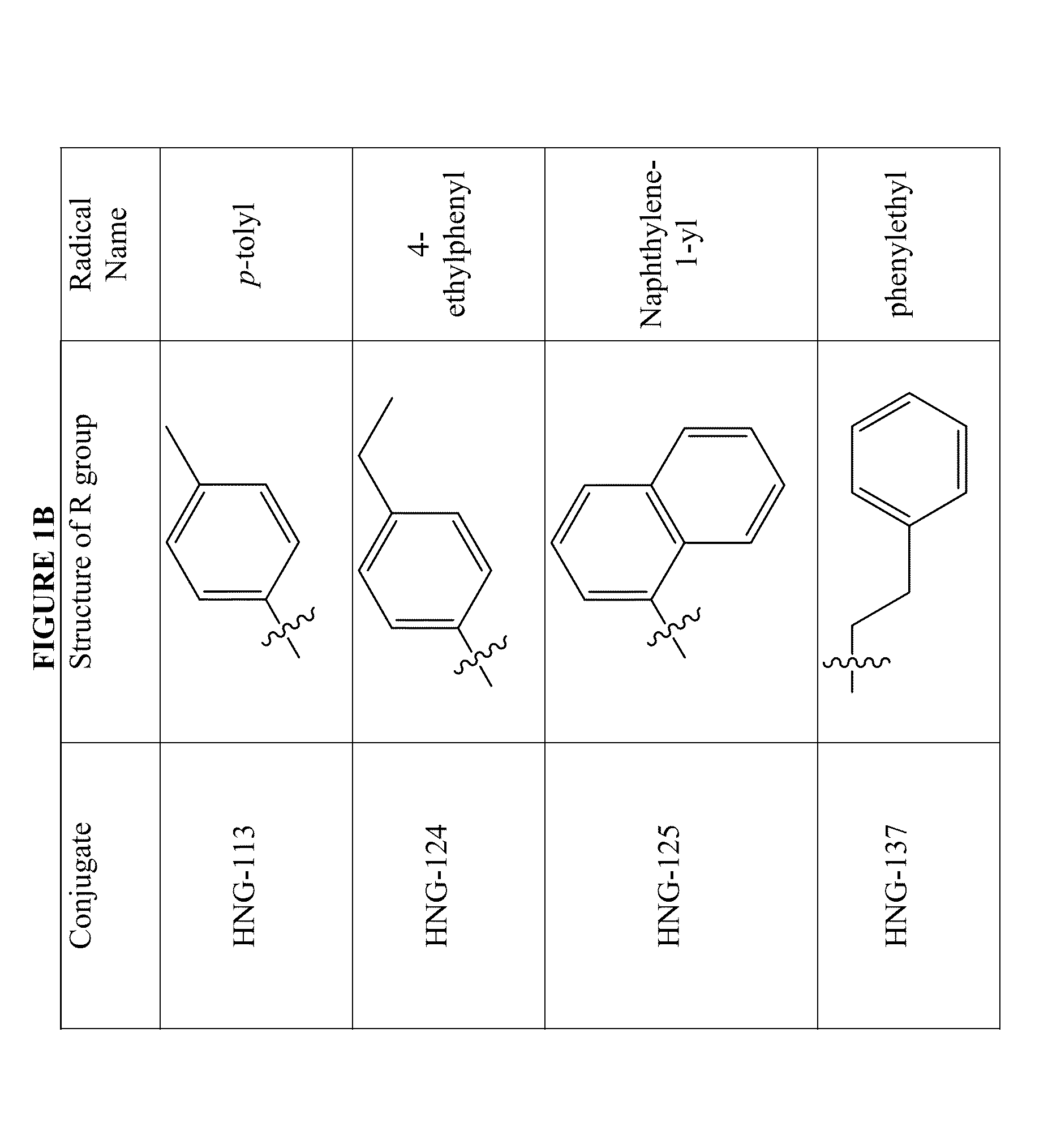
Patents
Literature
54 results about "Entry inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Entry inhibitors, also known as fusion inhibitors, are a class of antiretroviral drugs, used in combination therapy for the treatment of HIV infection. This class of drugs interferes with the binding, fusion and entry of an HIV virion to a human cell. By blocking this step in HIV's replication cycle, such agents slow the progression from HIV infection to AIDS.
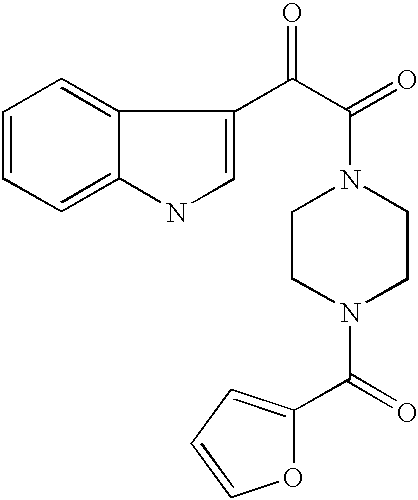
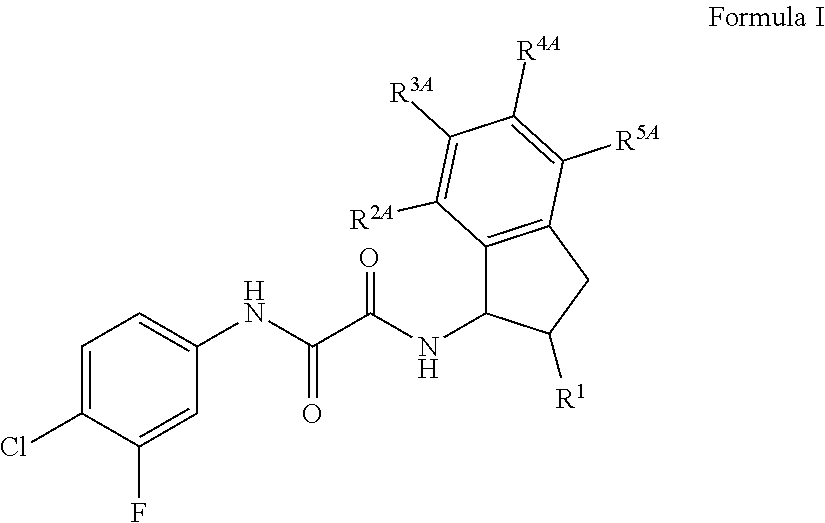
Antiviral indoleoxoacetyl piperazine derivatives
This invention provides compounds having drug and bio-affecting properties, their pharmaceutical compositions and method of use. In particular, the invention is concerned with indoleoxoacetyl piperazine derivatives. These compounds possess unique antiviral activity, whether used alone or in combination with other antivirals, antiinfectives, immunomodulators or HIV entry inhibitors. More particularly, the present invention relates to the treatment of HIV and AIDS.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Composition and antiviral activity of substituted azaindoleoxoacetic piperazine derivatives
This invention provides compounds having drug and bio-affecting properties, their pharmaceutical compositions and method of use. In particular, the invention is concerned with azaindoleoxoacetyl piperazine derivatives. These compounds possess unique antiviral activity, whether used alone or in combination with other antivirals, antiinfectives, immunomodulators or HIV entry inhibitors. More particularly, the present invention relates to the treatment of HIV and AIDS.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
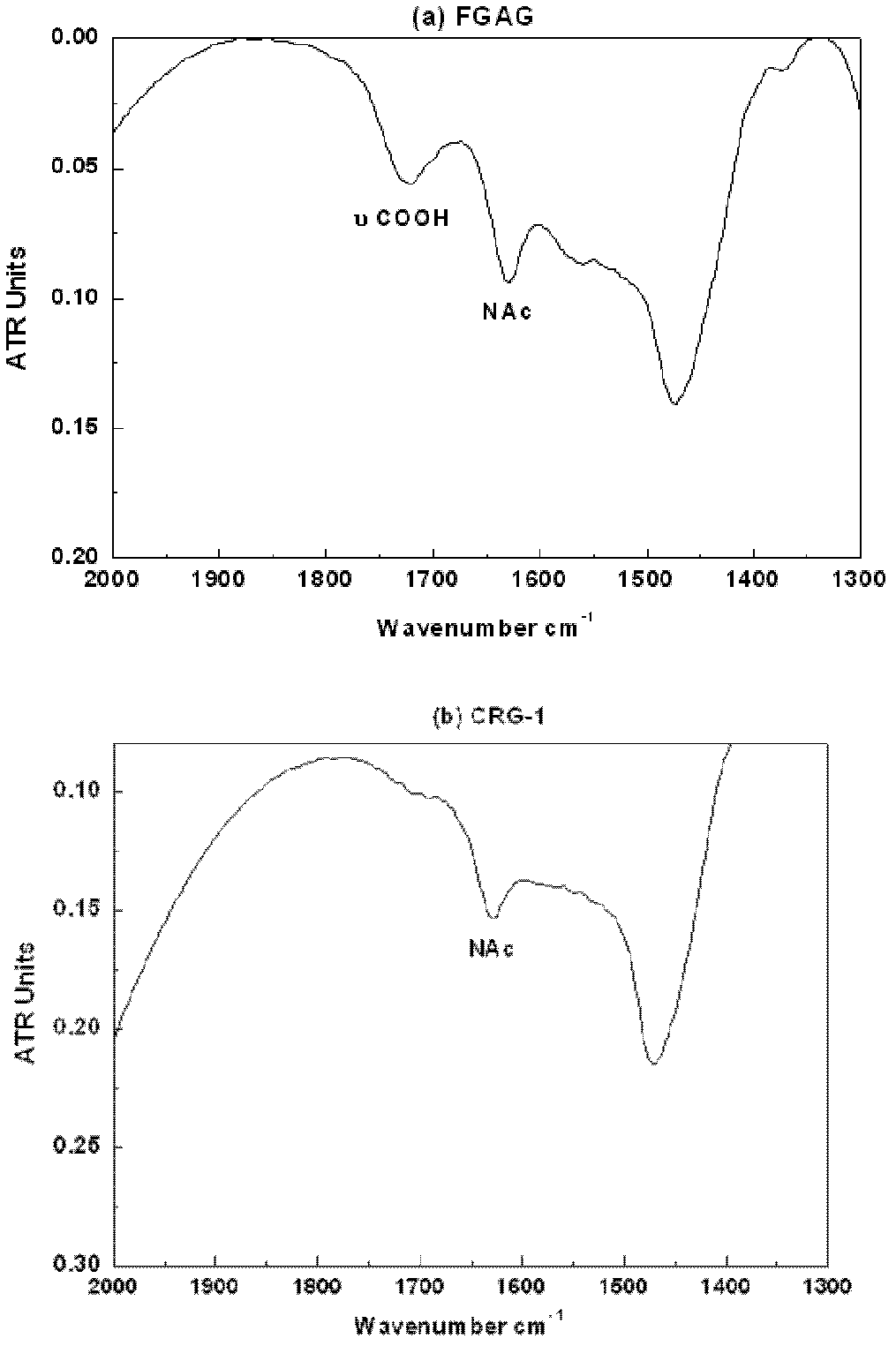
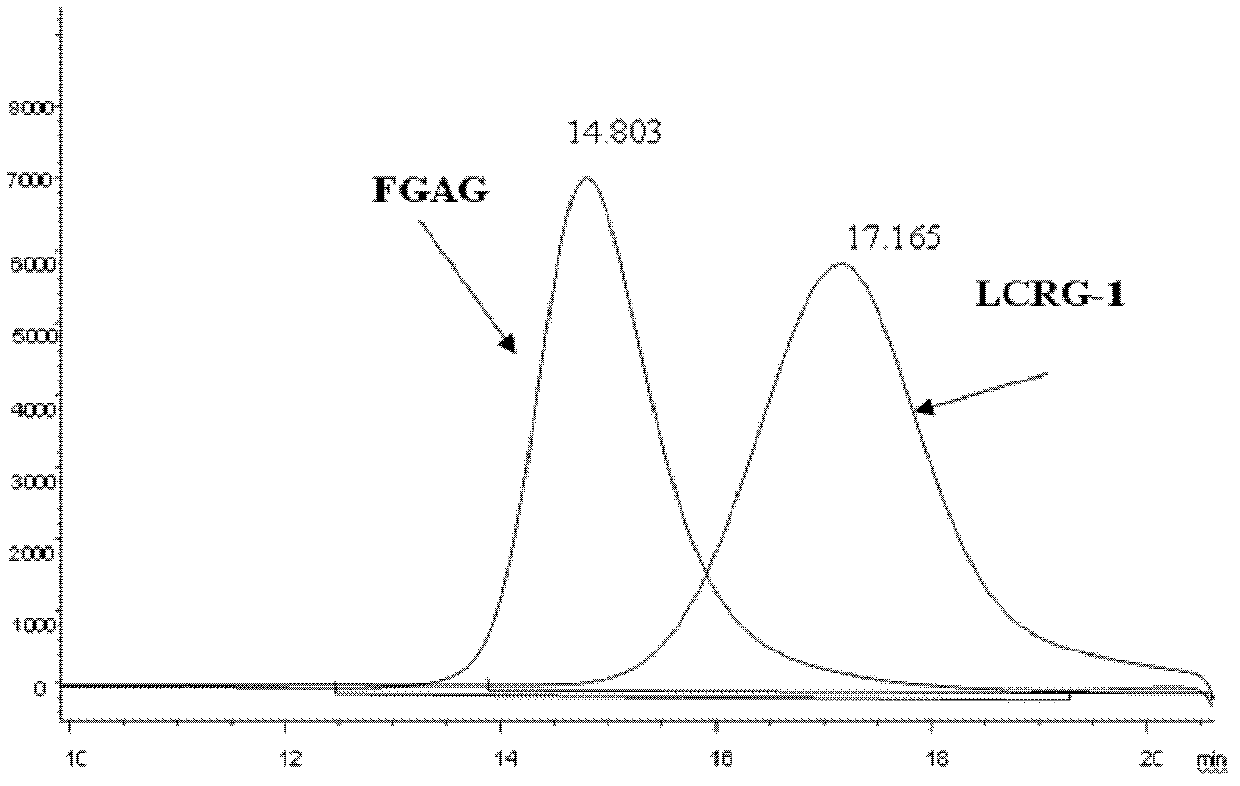
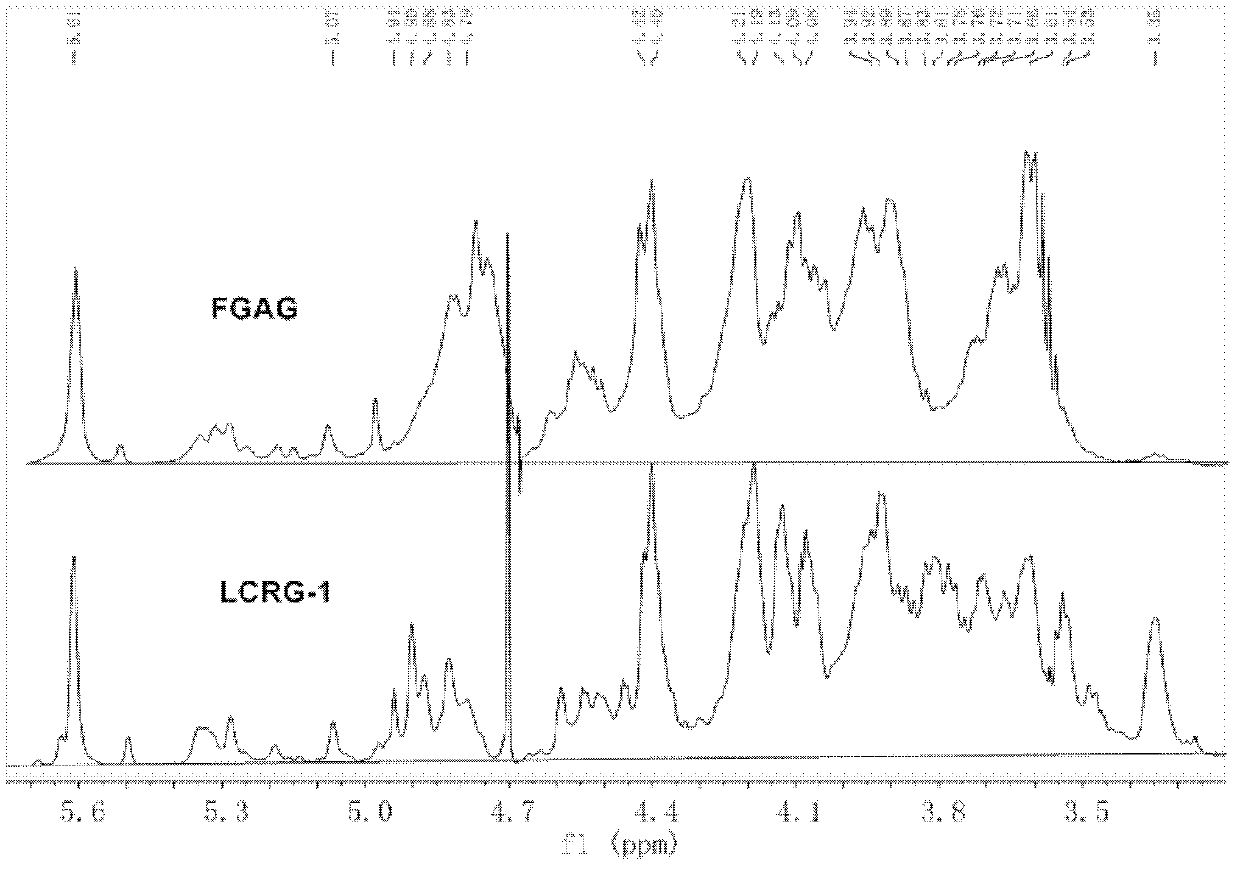
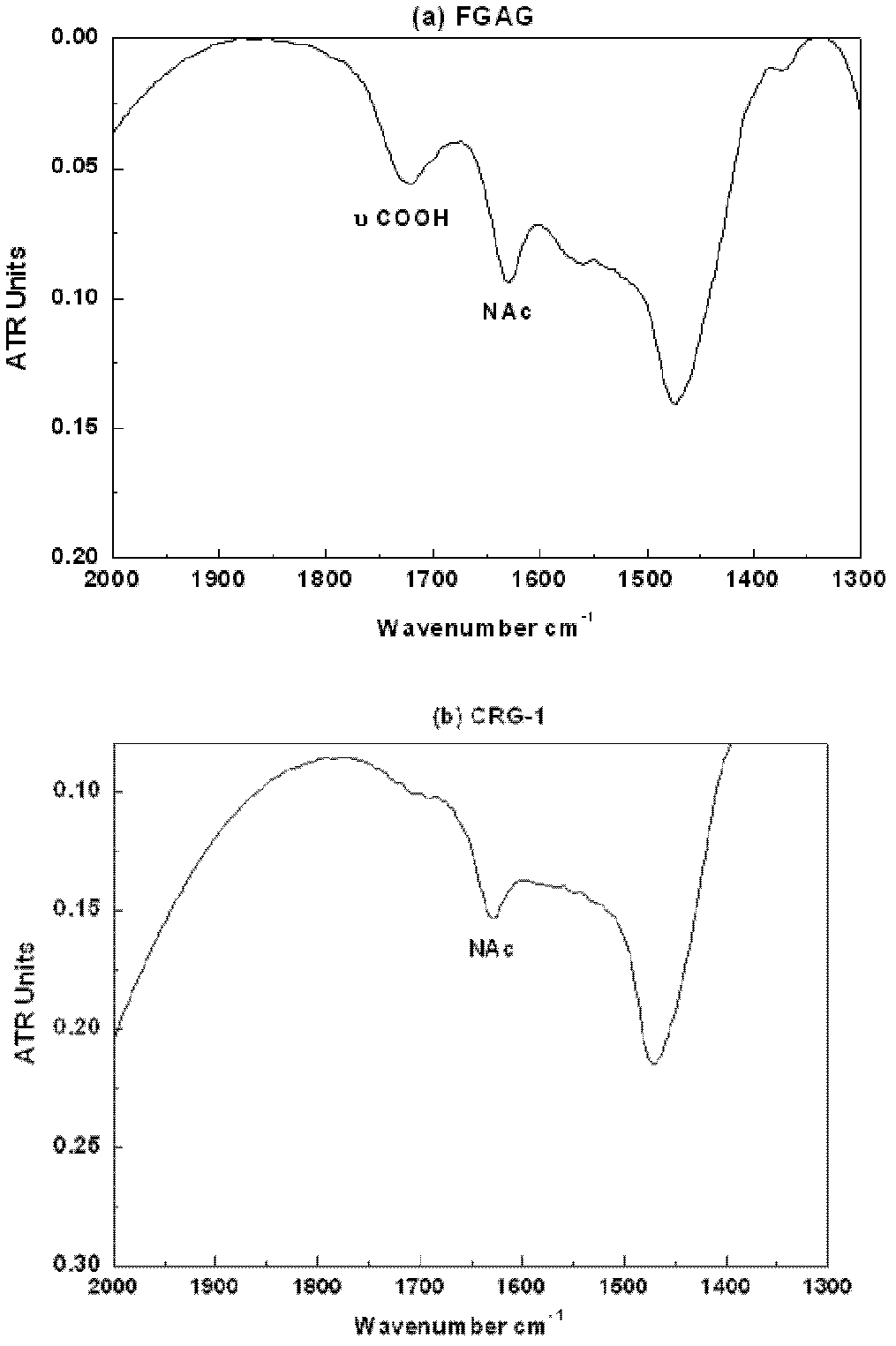
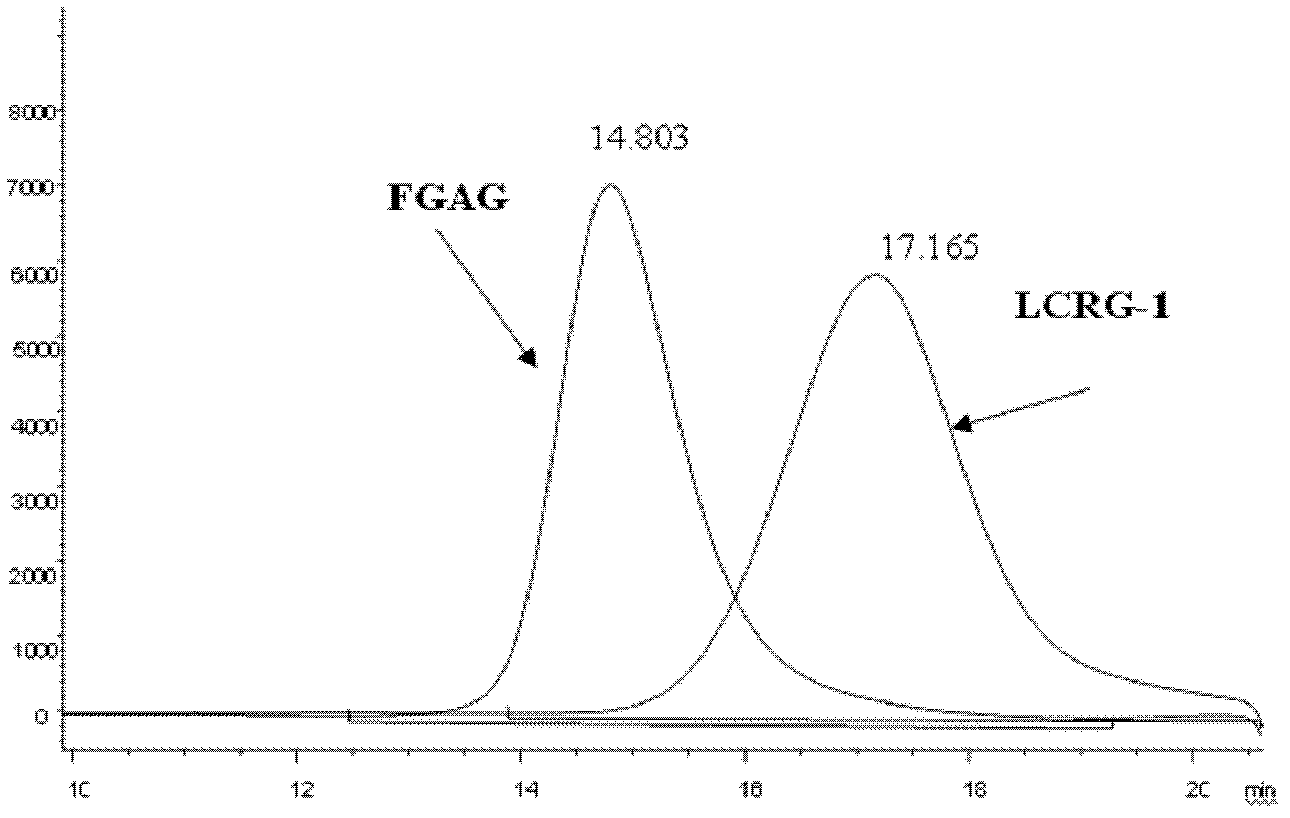
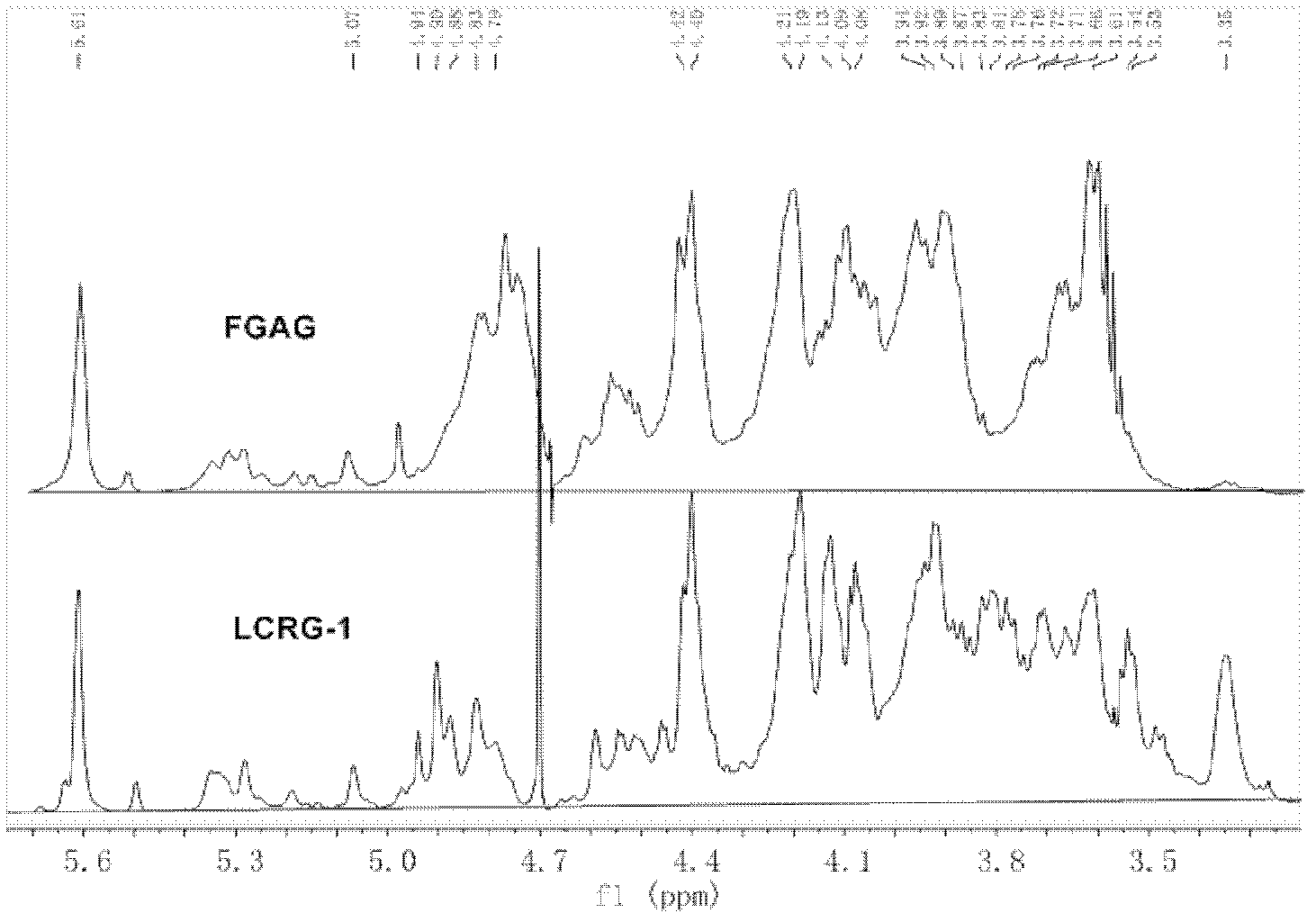
Low molecular weight carboxyl-reduced derivatives of fucosylated glycosaminoglycans and preparation method and applications of low molecular weight carboxyl-reduced derivatives
The invention discloses low molecular weight carboxyl-reduced derivatives of fucosylated glycosaminoglycans (LCRG). The extent of carboxyl reduction is not less than 20%. Weight-average molecular weight of the LCRG is about 3000-20000Da, and monosaccharides comprise acetyl galactosamine (GalNAc), glucose (Glc) or glucuronic acid (GlcUA) and fucose (Fuc) or sulfates of fucose (shown as -OSO3-). The mole ratio of GalNAc, Glc (containing GlcUA), Fuc and -OSO3- is about 1: (1+-0.3): (1+-0.3): (3.0+-1.0). The LCRG is a potent human immunodeficiency virus (HIV) Type 1 entry inhibitor which acts on conserved regions and has the advantages of high activity against HIV Type 1, high therapeutic index and no-drug-resistance, and the LCRG can be used for preventing or curing HIV. The invention further provides a preparation method of the LCRG. The carboxyl-reduced derivatives of fucosylated glycosaminoglycans and medicinal compositions of the carboxyl-reduced derivatives can be prepared into injection agents, lyophilized powder or suppository and the like.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
Indole, azaindole and related heterocyclic N-substituted piperazine derivatives
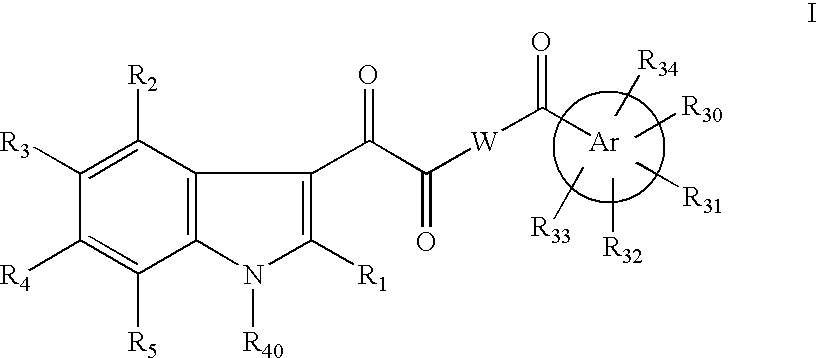
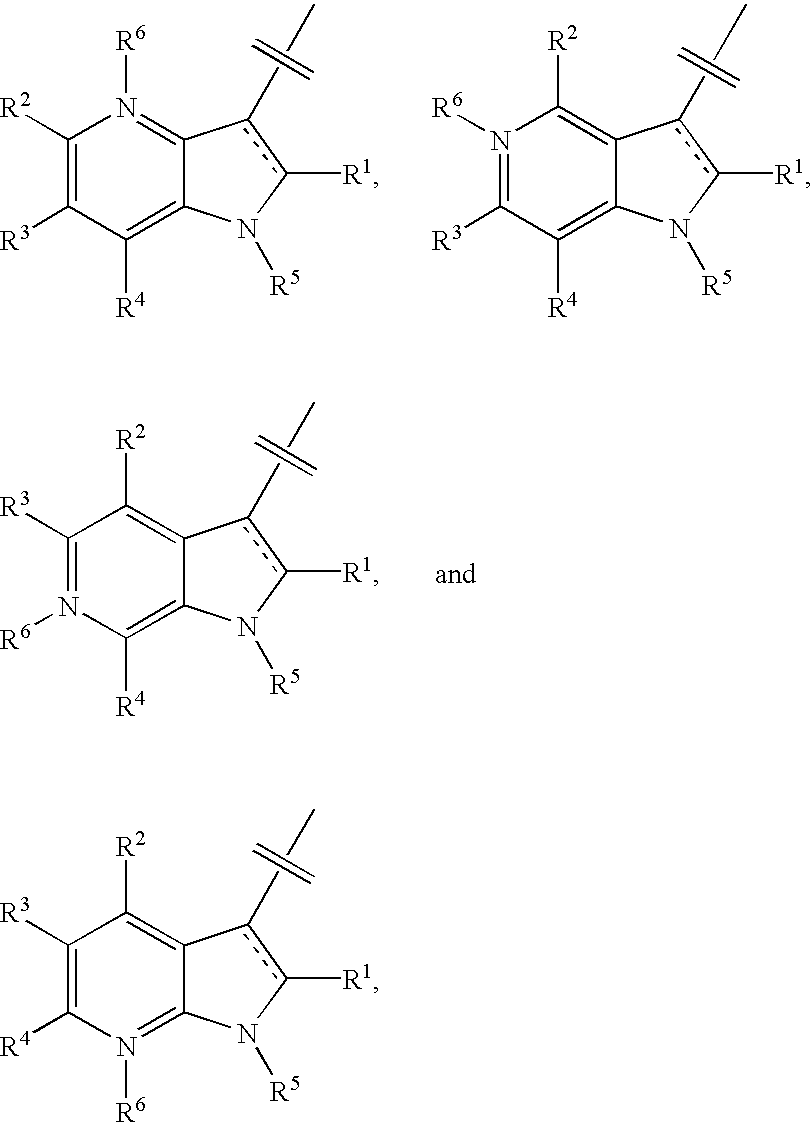
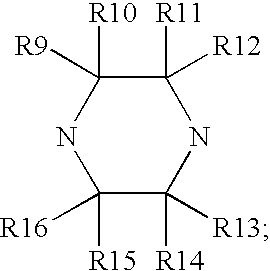
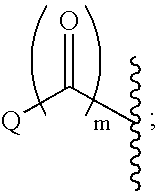
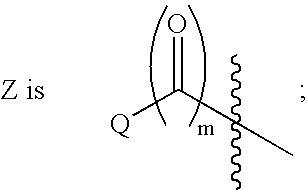
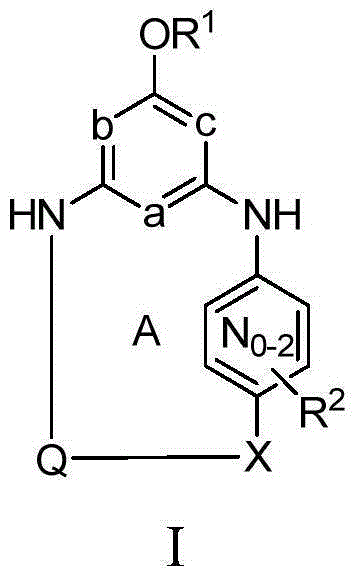
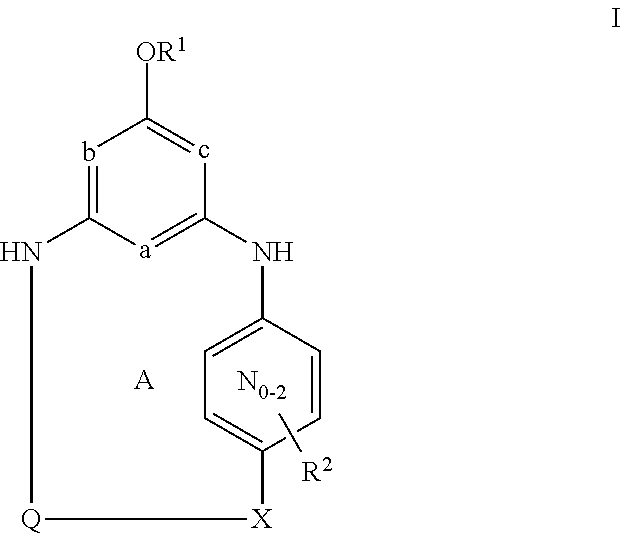
This invention provides compounds of Formula I, including pharmaceutically accceptable salts thereof, having drug and bio-affecting properties, their pharmaceutical compositions and method of use. These compounds possess unique antiviral activity, whether used alone or in combination with other antivirals, antiinfectives, immunomodulators or HIV entry inhibitors. More particularly, the present invention relates to the treatment of HIV and AIDS. The compounds of Formula I have the formula wherein: Z is Q is selected from the group consisting of m is 2; A is selected from the group consisting of cinnolinyl, napthyridinyl, quinoxalinyl, pyridinyl, pyrimidinyl, quinolinyl, isoquinolinyl, quinazolinyl, azabenzofuryl, and phthalazinyl each of which may be optionally substituted with one or two groups independently selected from methyl, methoxy, hydroxy, amino and halogen; and —W— is
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Low molecular weight glycosylated chondroitin sulfate and its purpose in preparation of anti-HIV-1 medicament
InactiveCN102247401AAvoid drug resistanceDepolymerization speed is stableOrganic active ingredientsAntiviralsMonosaccharide compositionUltrafiltration
The invention discloses a low molecular weight glycosylated chondroitin sulfate, whose weight average molecular weight is 3000-15000Da. The monosaccharide composition comprises acetyl galactosamine (D-GalNAc), glucuronic acid (D-GlcUA), fucose (L-Fuc), or its sulfuric ester (expressed in -OS03<->), wherein the mole ratio of D-GalNAc to D-GlcUA to L-Fuc to -OS03<-> is 1: (1+ / -0.3): (1+ / -0.3): (3.5+ / -0.5). The low molecular weight glycosylated chondroitin sulfate has a strong anti-HIV-1 virus activity, is a gp120 entry inhibitor, and can be used for preventing and / or treating AIDS. The invention also provides a method for preparing the low molecular weight glycosylated chondroitin sulfate and its composition preparation. Glycosylated chondroitin sulfate is depolymerized by the peroxide method to obtain a low molecular weight product, and then low molecular and / or high-molecular impurities of the product are removed by gel separation or ultrafiltration method. The low molecular weight glycosylated chondroitin sulfate and its medicinal composition can be prepared in the form of an injection, a lyophilized powder or a suppository.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
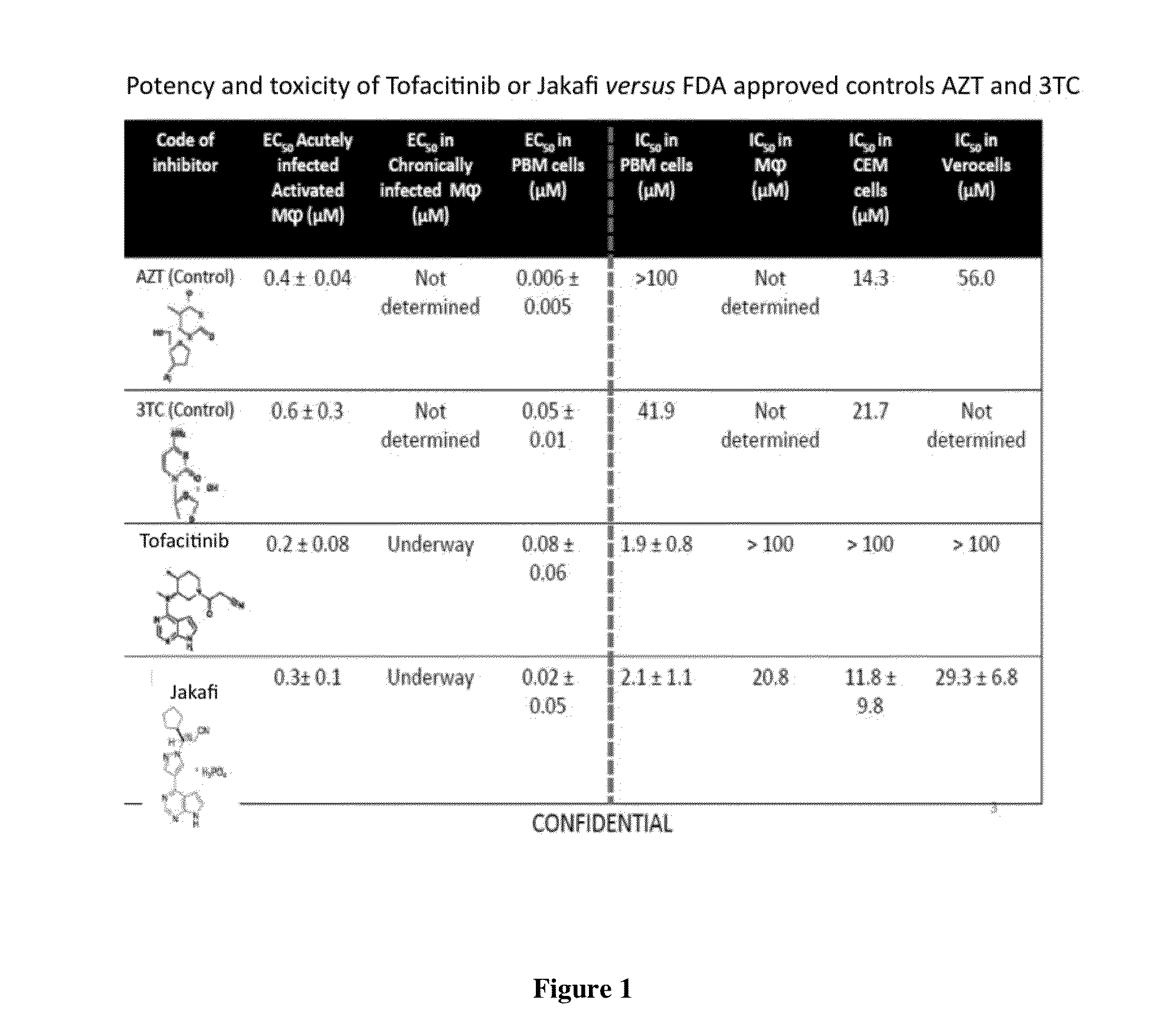
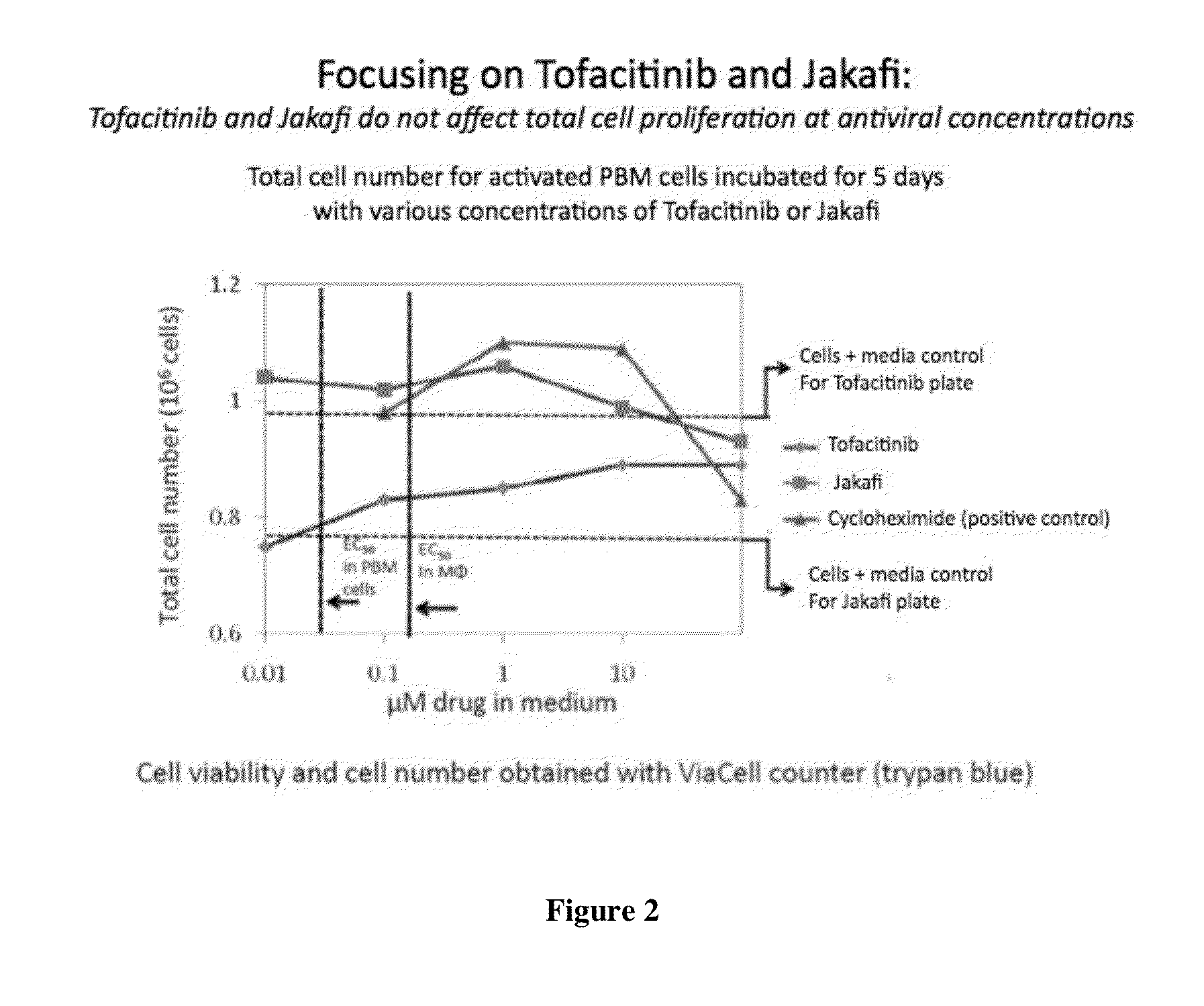
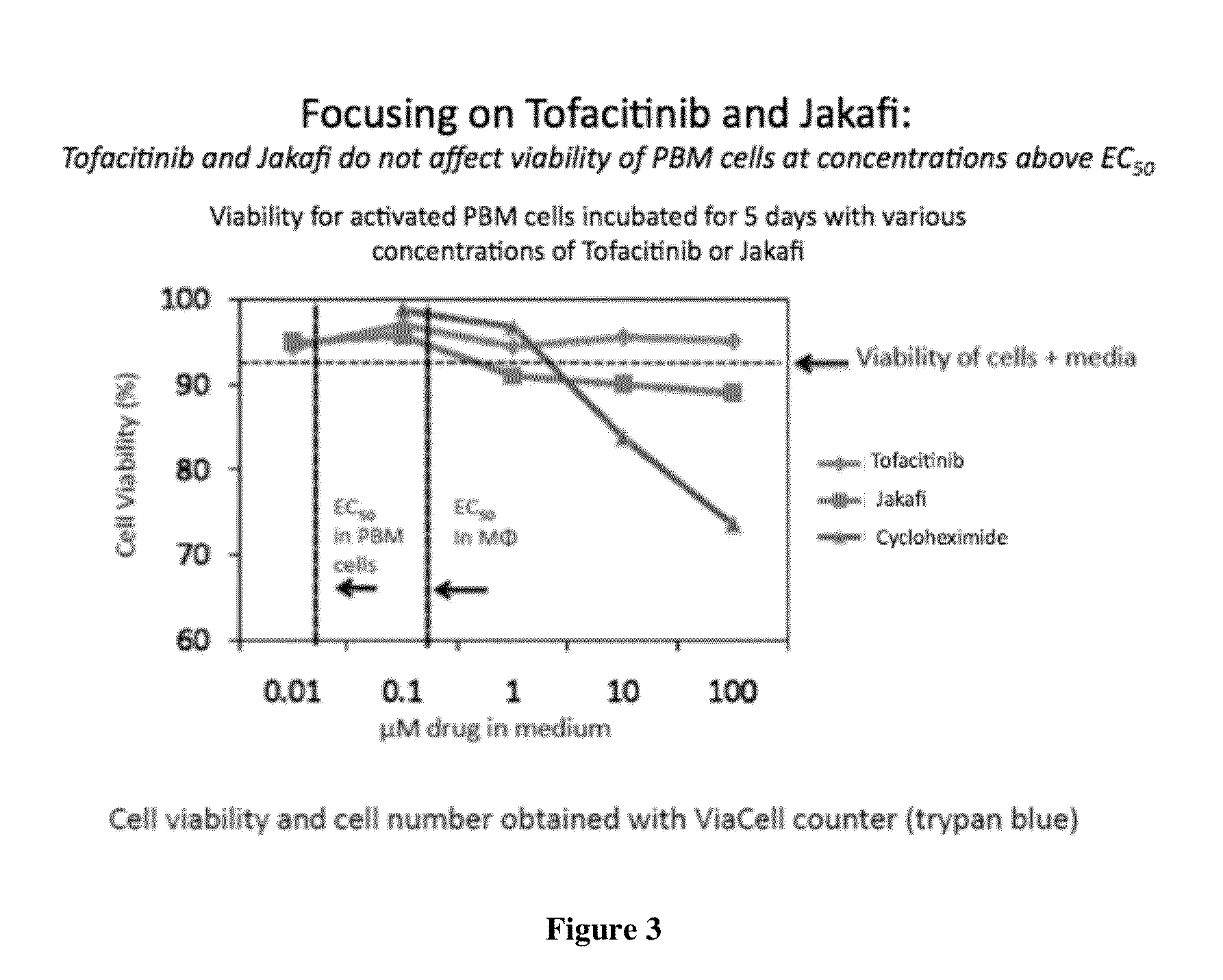
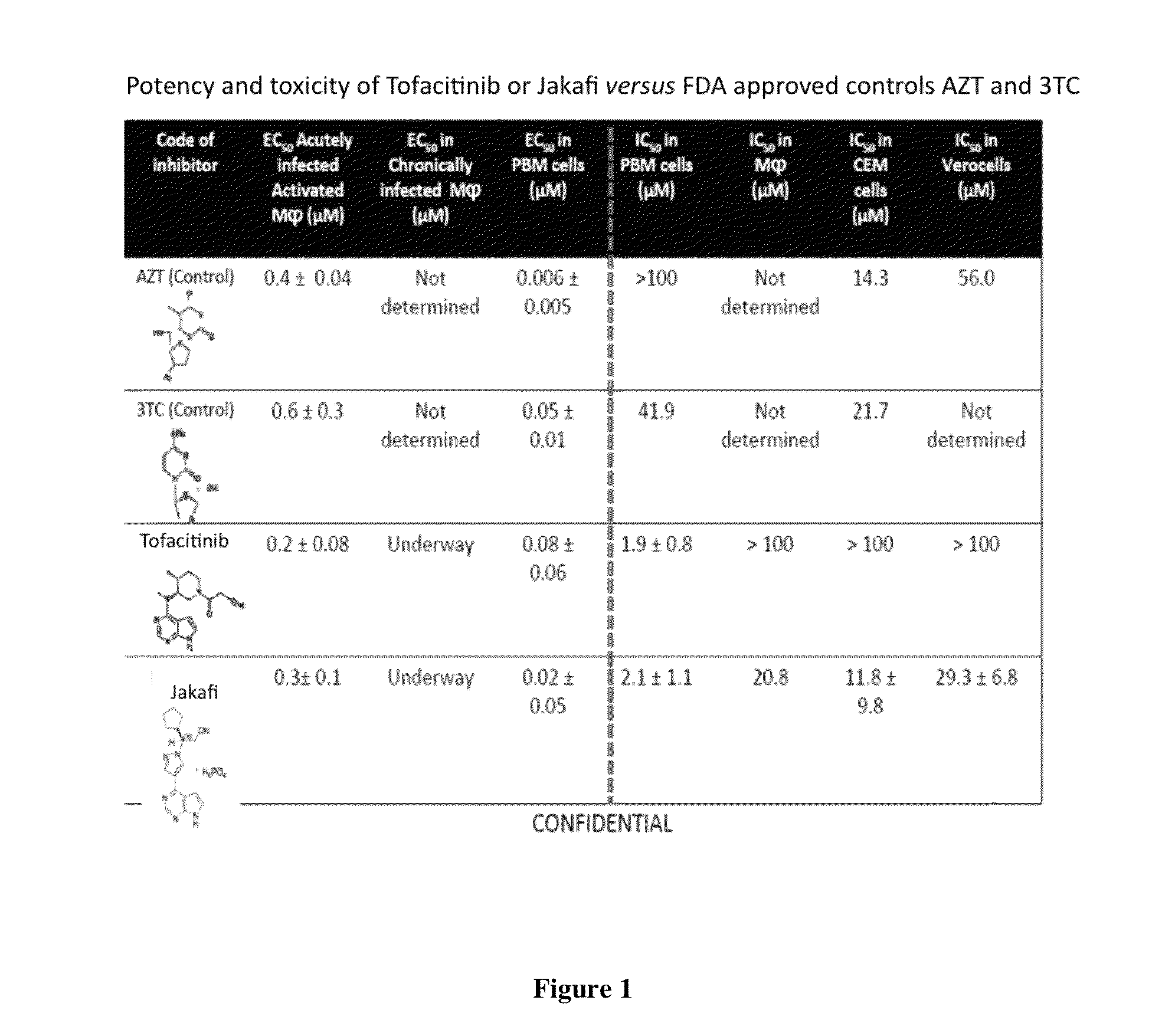
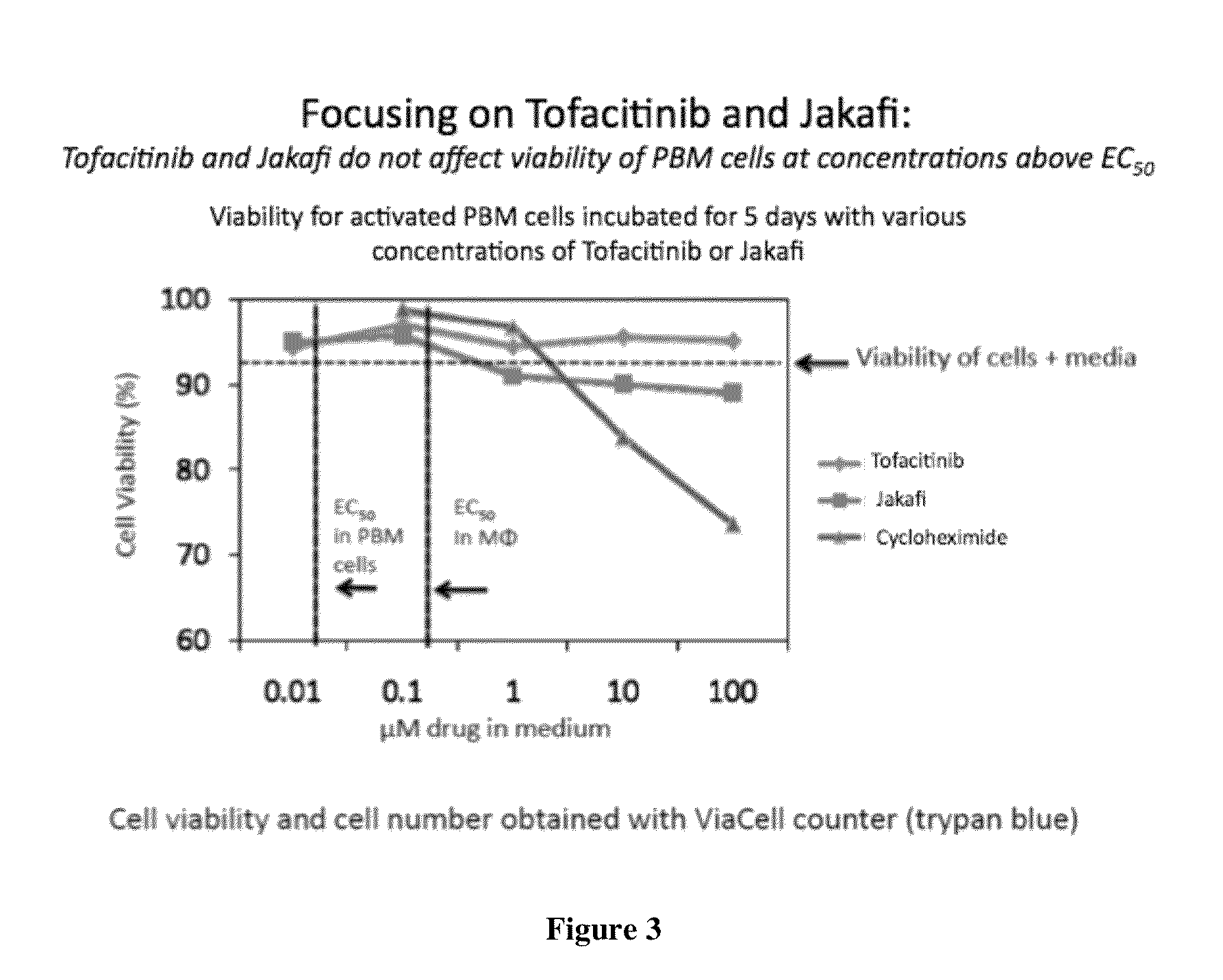
Antiviral jak inhibitors useful in treating or preventing retroviral and other viral infections
ActiveUS20140328793A1Improve their absolute antiviral effectLow toxicityBiocidePeptide/protein ingredientsProteinase inhibitorThymidine
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Methods and compositions for the inhibition of HIV infection of t cells
InactiveUS20090053220A1Inhibit bindingAntiviralsAntibody ingredientsAntiendomysial antibodiesSoluble CD4
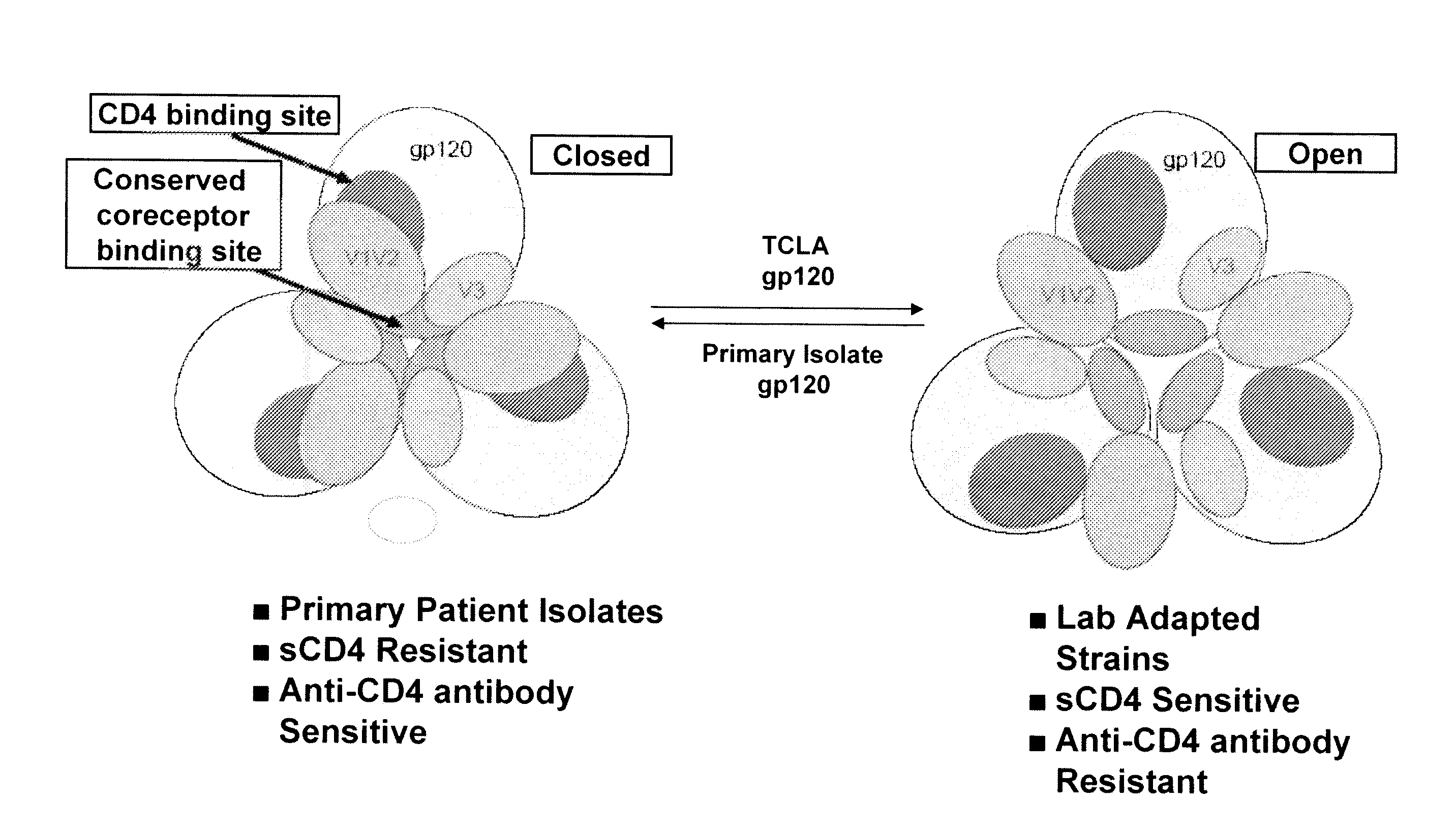
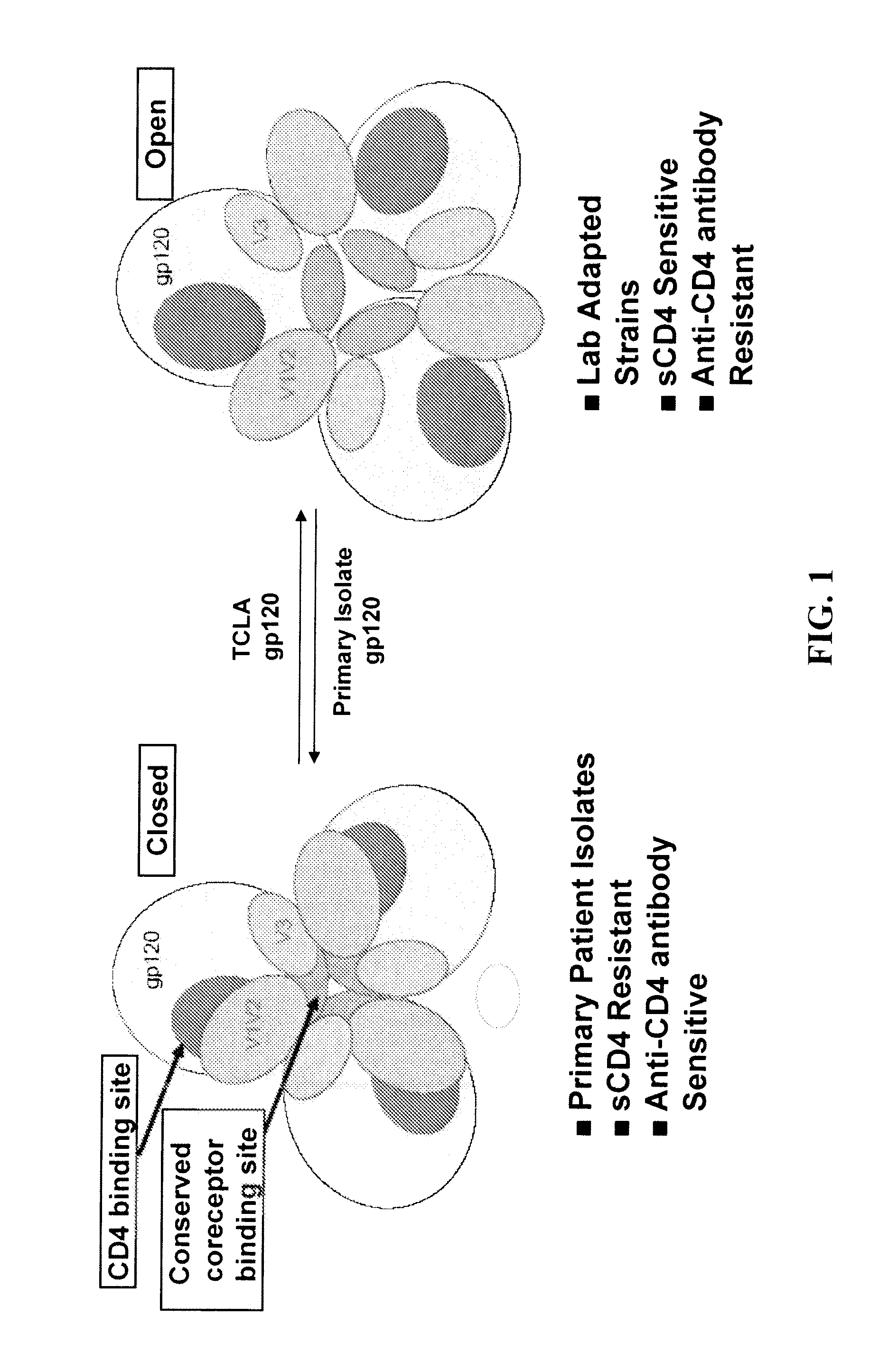
The present invention is based upon the surprising discovery that exposure of a non-resistant HIV to a first entry inhibitor, such as an anti-CD4 antibody or a co-receptor inhibitor, which like all current HIV drugs selects for mutations that result in a resistant HIV, surprisingly results in HIV viruses much more susceptible to neutralization by a second entry inhibitor, such as soluble CD4 (sCD4) or an HIV gp41 inhibitor. Therefore, the present invention provides methods and compositions for inhibiting HIV-1 infection in a subject that overcomes the problem of drug resistance.
Owner:GENENTECH INC
Antiviral JAK inhibitors useful in treating or preventing retroviral and other viral infections
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Inhibitors of filovirus entry into host cells
Organic compounds showing the ability to inhibit viral glycoprotein (GP)-mediated entry of a filovirus into a host cell are disclosed. The disclosed filovirus entry inhibitor compounds are useful for treating, preventing, or reducing the spread of infections by filovirus including the type species Marburg virus (MARV) and Ebola virus (EBOV). Preferred inhibitors of the invention provide therapeutic agents for combating the Ivory Coast, Sudan, Zaire, Bundibugyo, and Reston Ebola virus strains.
Owner:MICROBIOTIX
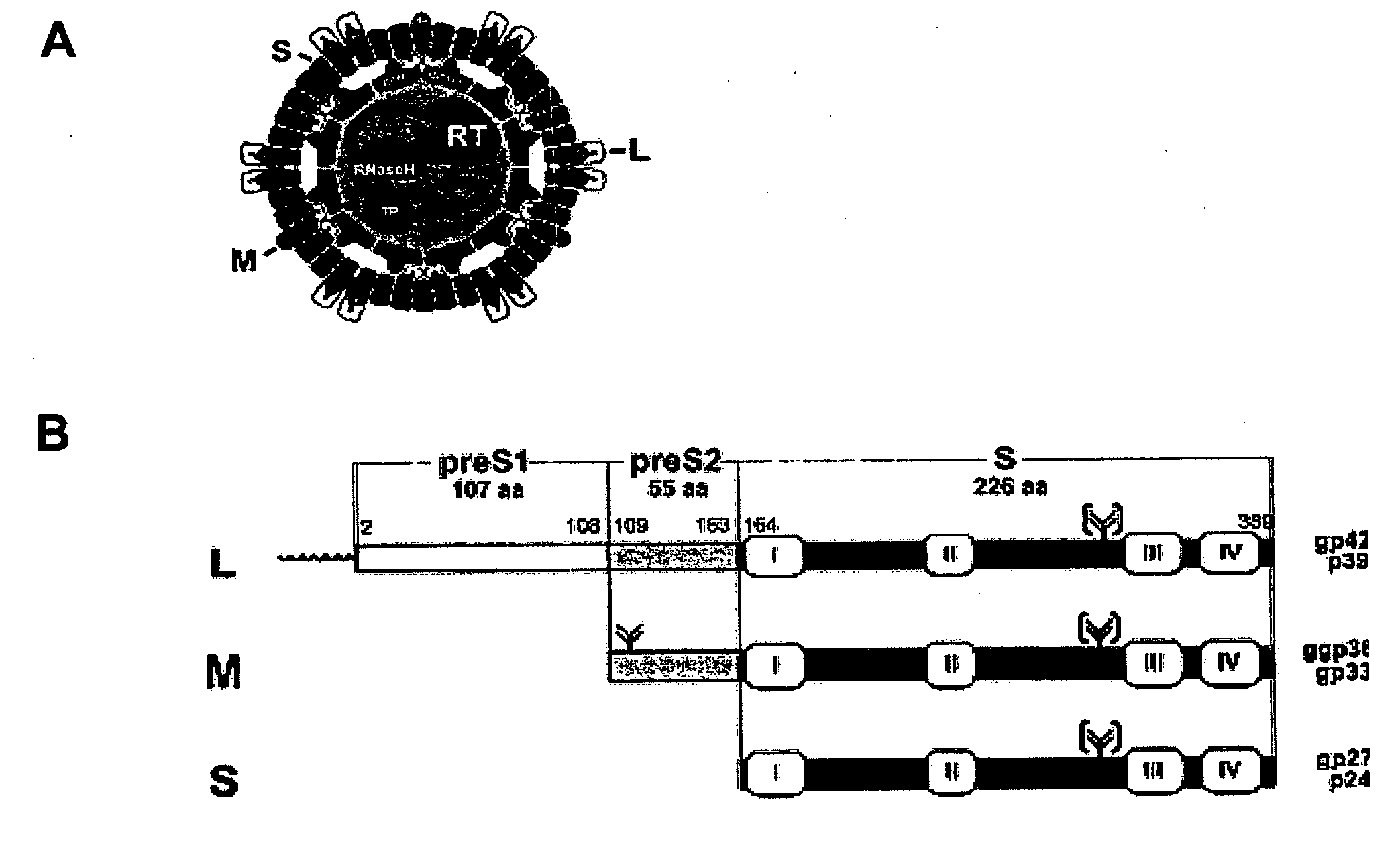
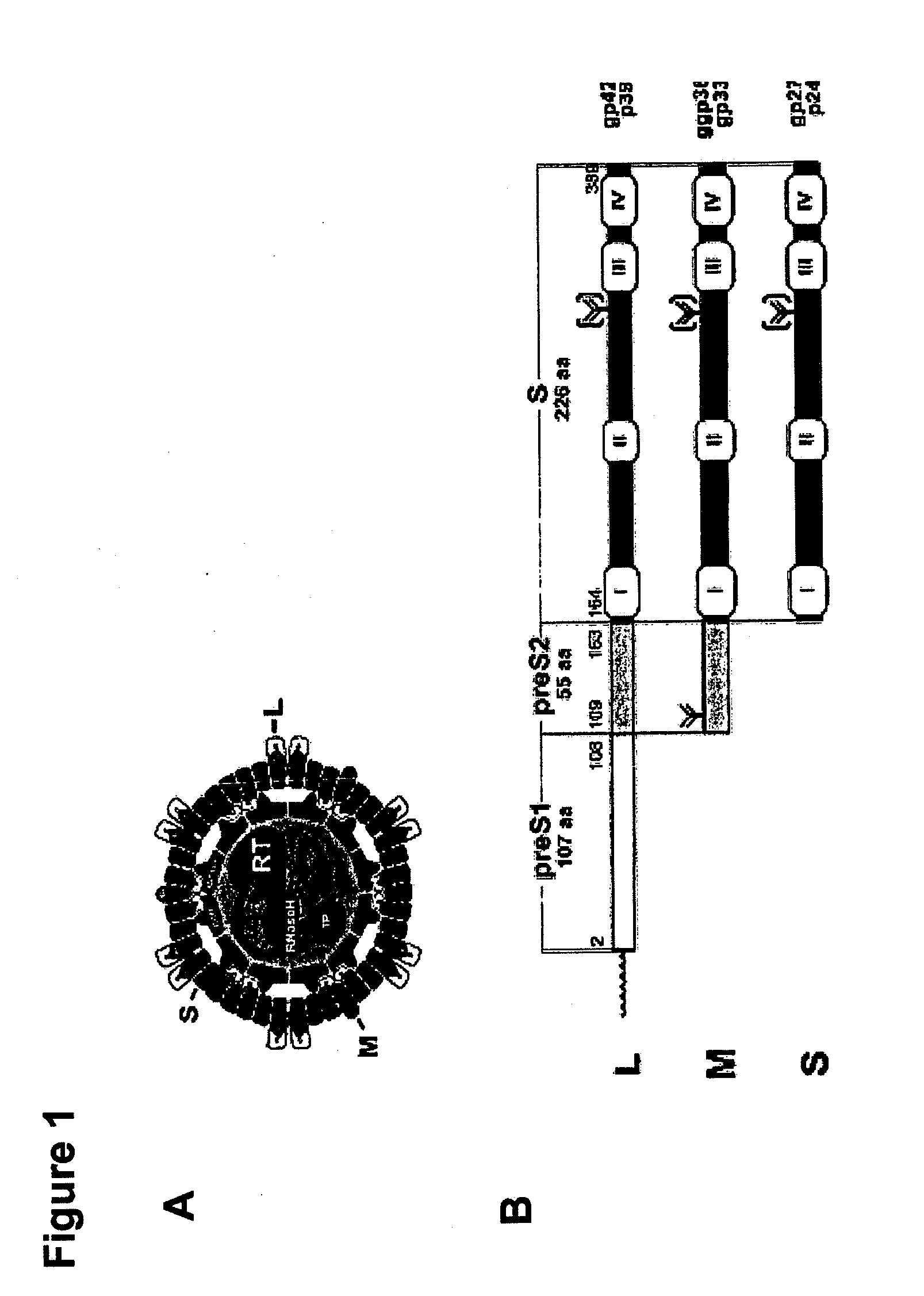
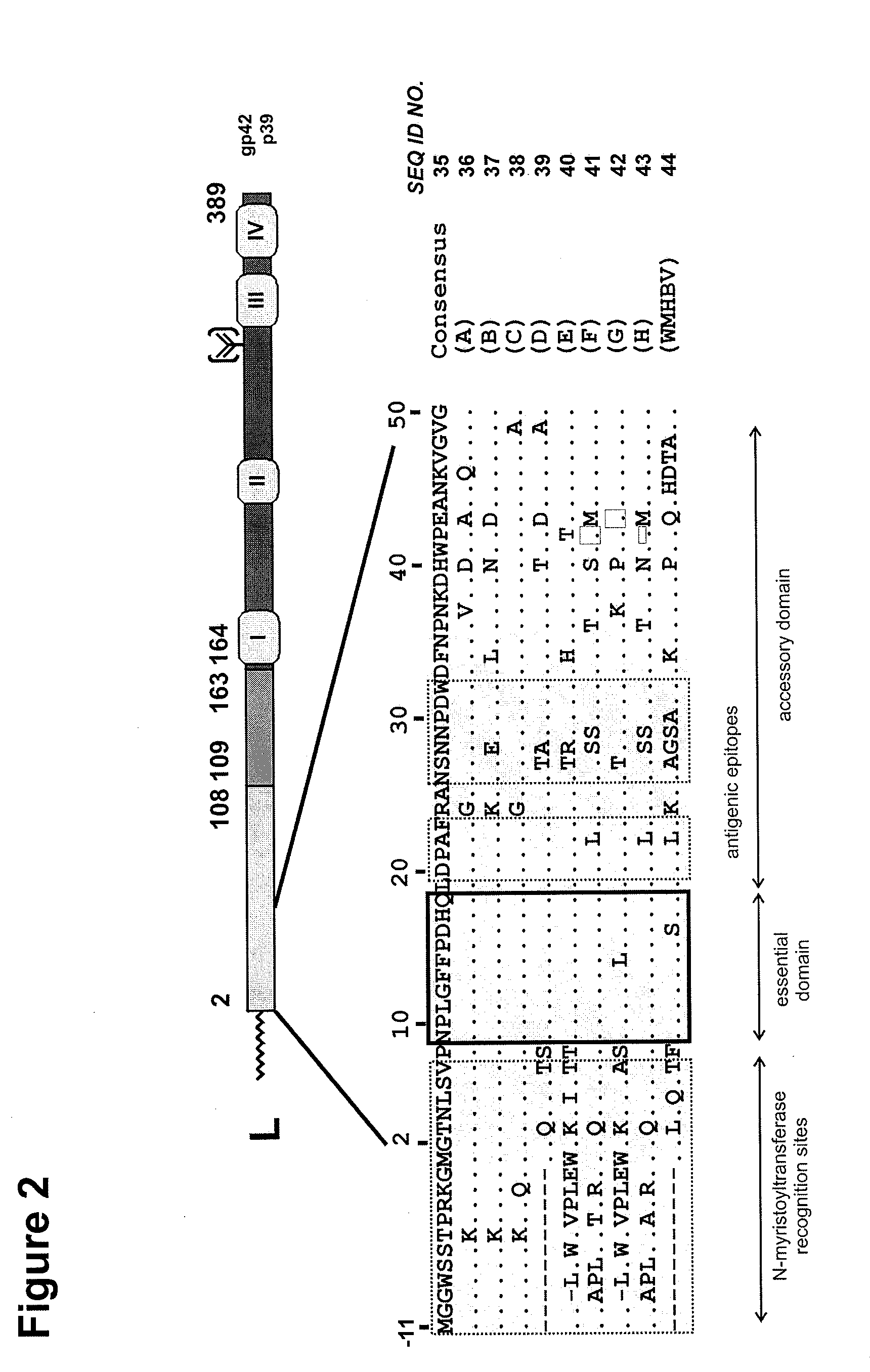
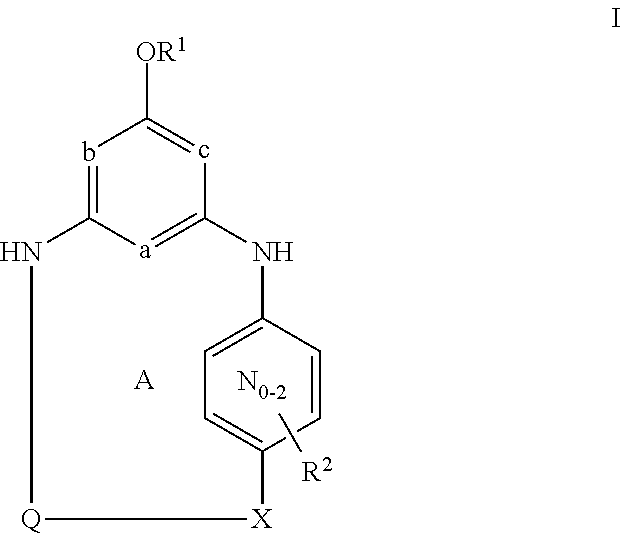
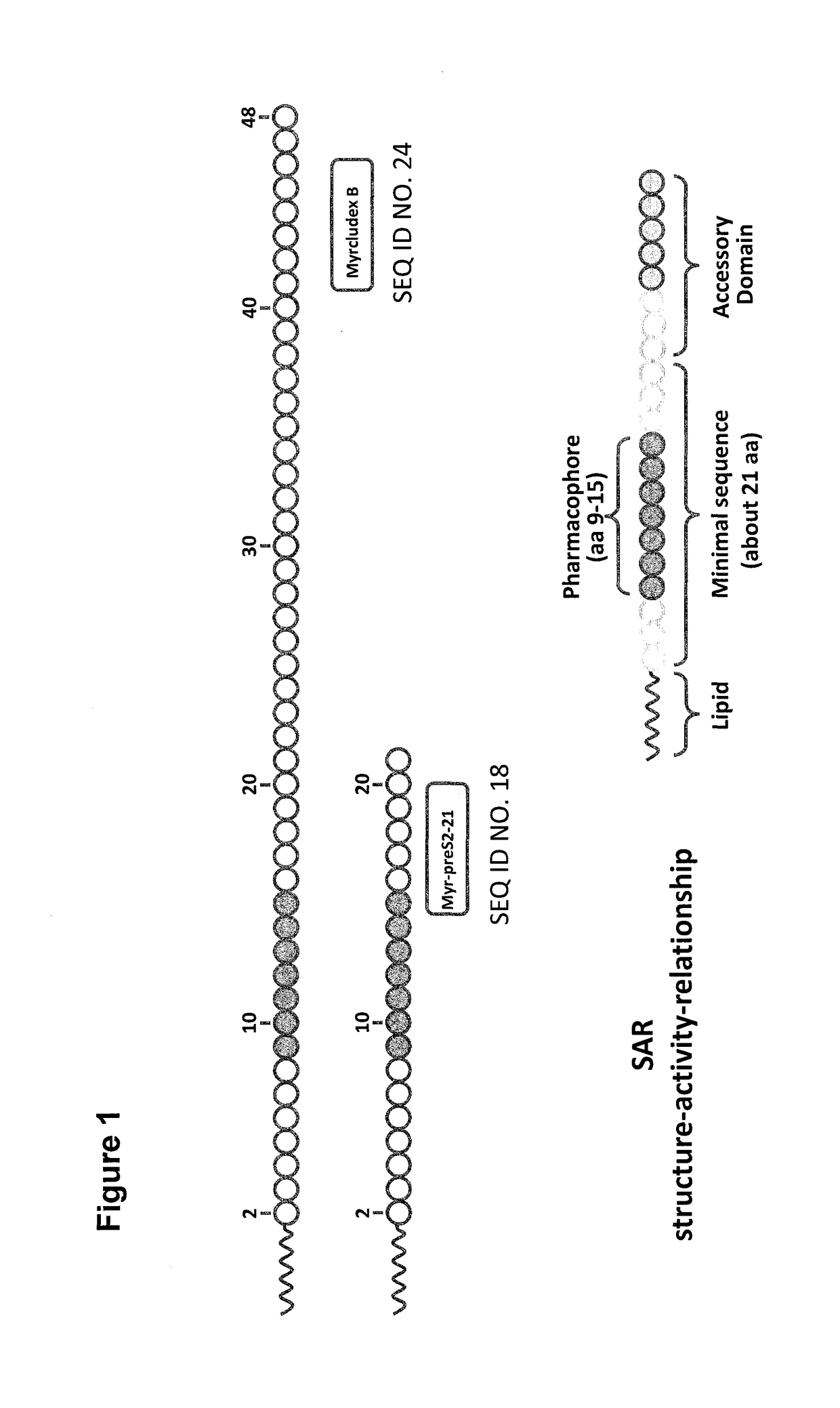
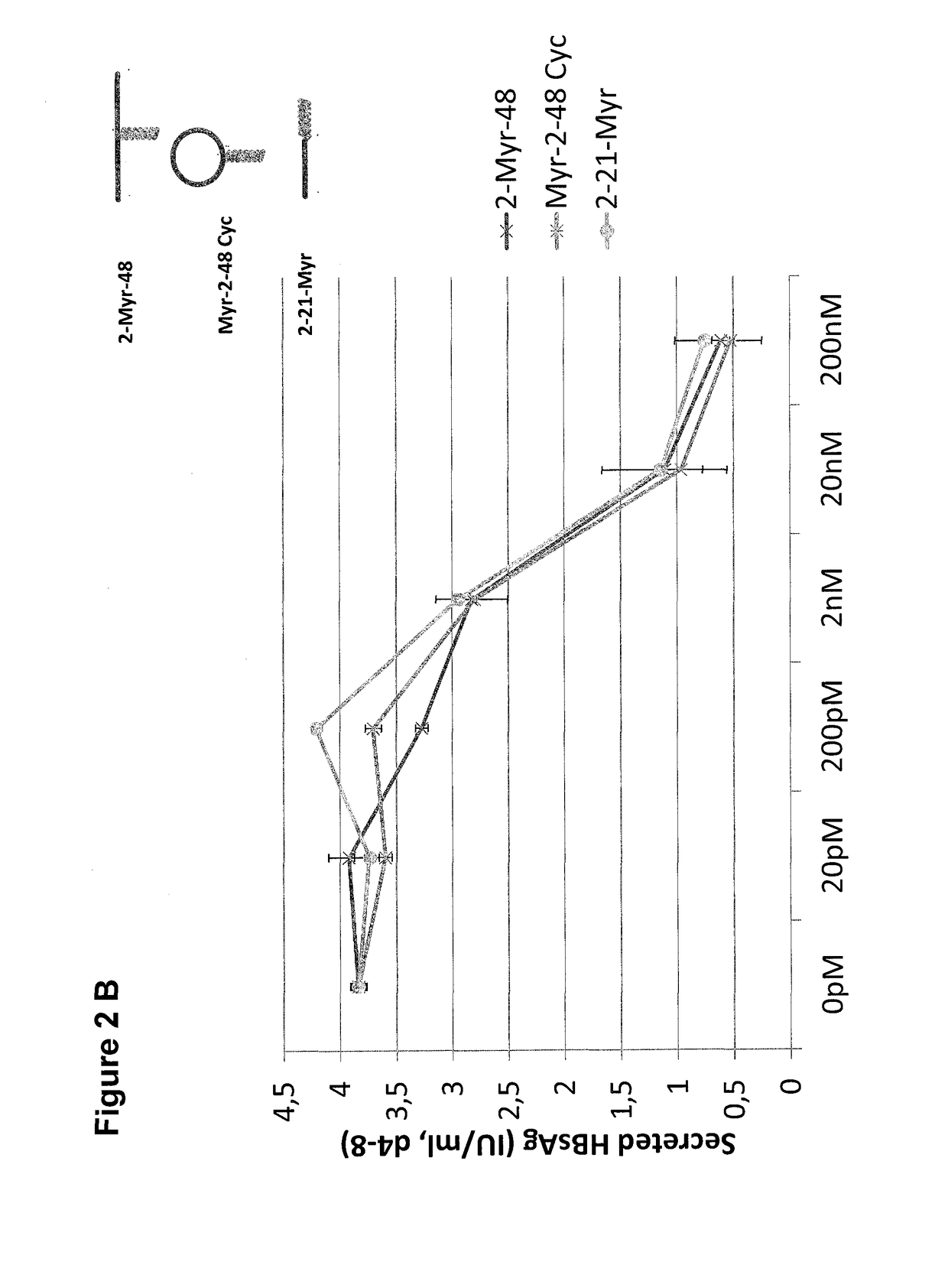
Hydrophobic modified pres-derived peptides of hepatitis b virus (HBV) and their use as hbv and hdv entry inhibitors
ActiveUS20110020397A1Avoid infectionPeptide/protein ingredientsViral antigen ingredientsHepatitis B virusHepacivirus
The present invention relates to hydrophobic modified preS-derived peptides of hepatitis B virus (HBV) which are derived from a HBV preS consensus sequence and are N-terminal preferably acylated and optional C-terminal modified. These hydrophobic modified preS-derived peptides of HBV are very effective HBV entry inhibitors as well as HDV entry inhibitors and are, thus, suitable for the inhibition of HBV and / or HDV infection, prevention of primary HBV and / or HDV infection as well as treatment of (chronic) hepatitis B and / or D. The present invention further relates to pharmaceutical and vaccine compositions comprising these hydrophobic modified preS-derived peptides of HBV.
Owner:UNIVERSITY OF HEIDELBERG
Novel in-vitro and in-vivo infection model based on Ebola virus-like particles
InactiveCN106636014AMicrobiological testing/measurementInactivation/attenuationBiosafety level 4Biological imaging
The invention provides a novel infection model based on EBOV (Ebola Virus) virus-like particles (VLP) and taking firefly luciferase (Fluc) as a reporter protein. The model enters cells with the help of an EBOV envelope protein (GP); the Fluc can be introduced into the cells after the model enters the cells, so that cell infection can be quantified according to signal intensity of the Fluc in the cells; mouse infection can be quantified through biological imaging according to signal intensity of bioluminescence in a mouse body. Therefore, the inventor can utilize the model to carry out in-vitro and in-vivo pre-screening experiments on a neutralizing antibody and an entered inhibitor of the GP outside a BSL-4 (Biosafety Level-4) laboratory.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Immunoassays, Haptens, Immunogens and Antibodies for Anti-HIV Therapeutics
InactiveUS20070218486A1Inhibits HIV propagationMicrobiological testing/measurementImmunoglobulinsNucleoside Reverse Transcriptase InhibitorProteinase activity
This invention provides compounds, methods, immunoassays, and kits relating to active, metabolically sensitive (“met-sensitive”) moieties of anti-HIV therapeutics, such as HIV protease inhibitors (PI), HIV nucleoside reverse transcriptase inhibitors (NRTI) and HIV entry inhibitors (EII).
Owner:ARK DIAGNOSTICS
Aglaroxin C and derivatives as HCV entry inhibitors
ActiveUS10085988B1Suppresses estrogen receptor (ER)-dependent gene activationOrganic chemistryAntibody ingredientsCancer researchHepatitis C virus
A rocaglamide or a rocaglate derivative, particularly aglaroxin C, blocks hepatitis C virus (HCV) entry with improved potency and therapeutic index.
Owner:TRUSTEES OF BOSTON UNIV +1
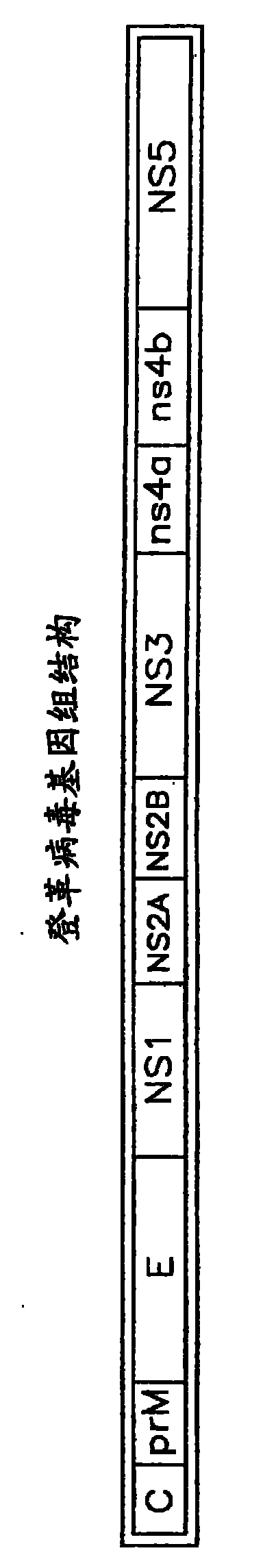
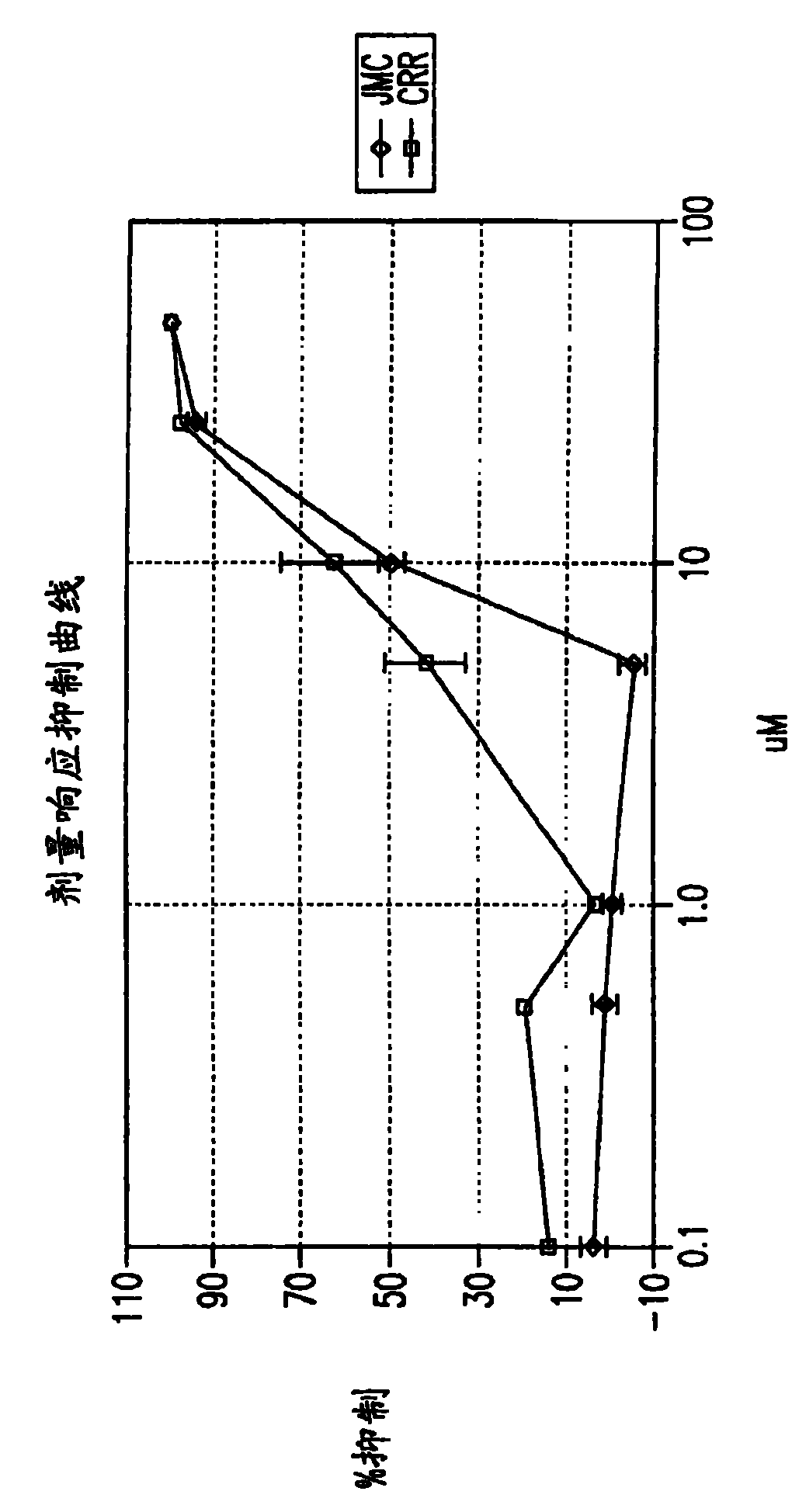
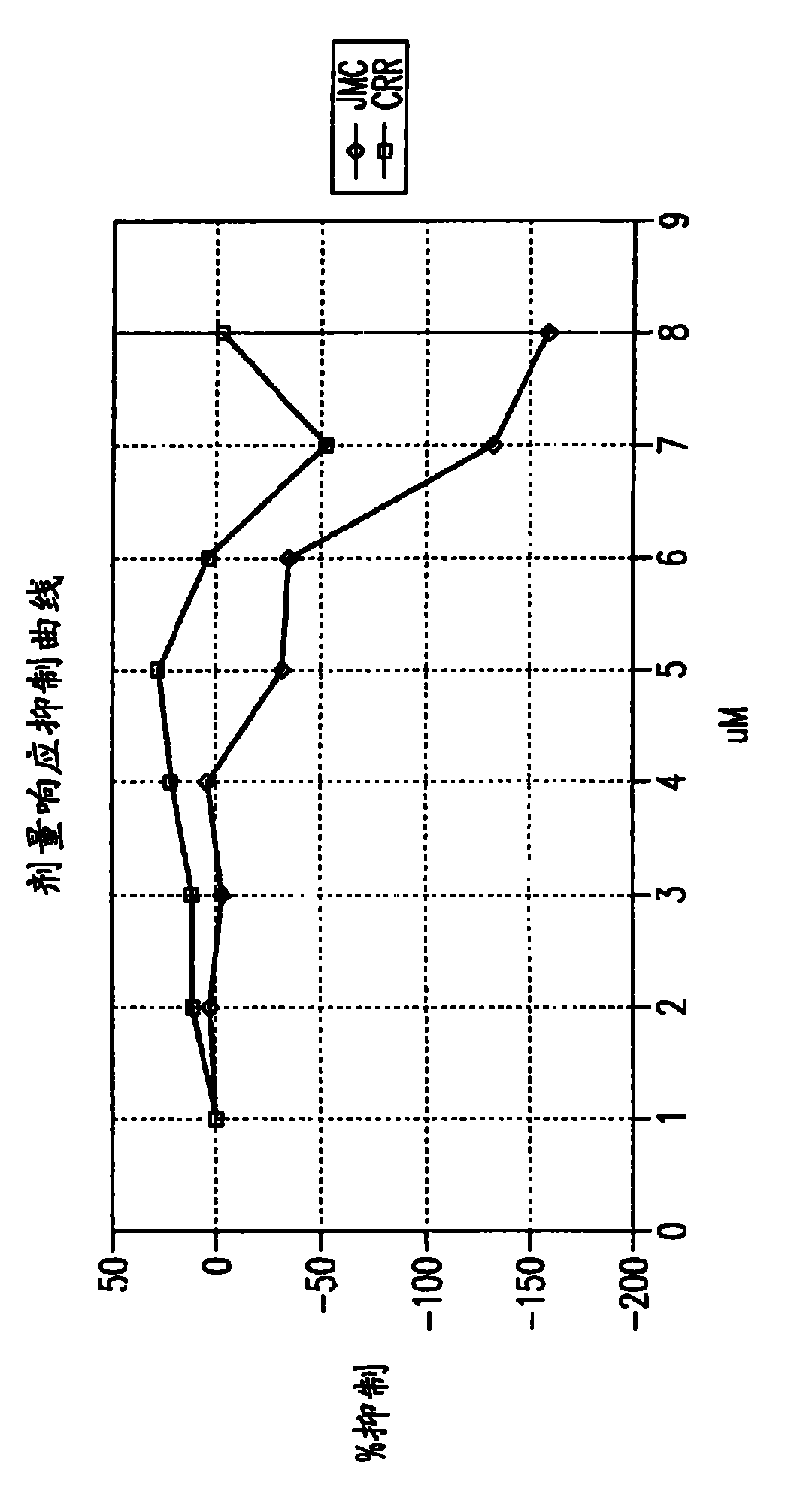
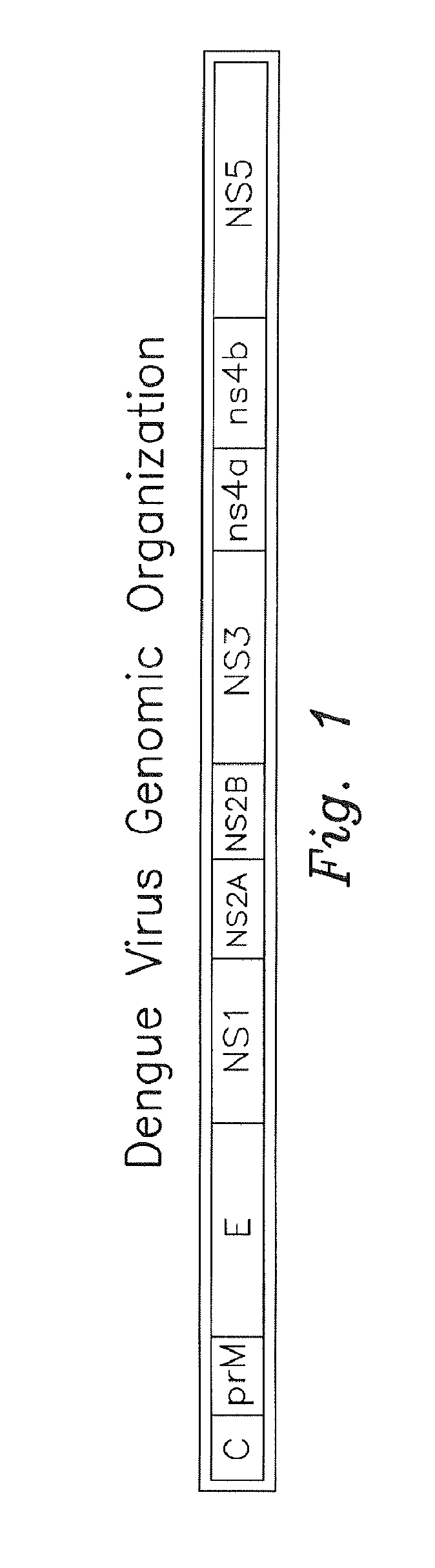
Optimized dengue virus entry inhibitory peptide (DN81)
Abstract The invention relates peptide entry inhibitors and methods of determining such inhibitors that are bindable to regions of viruses having class II E proteins, such as the dengue virus E protein, as candidates for in vivo anti-viral compounds.
Owner:FLORIDA GULF COAST UNIV BOARD OF TRUSTEES
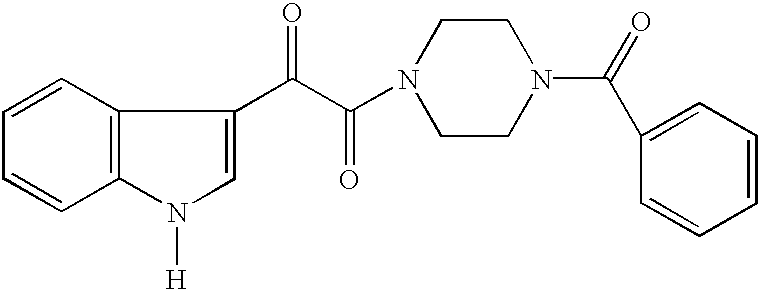
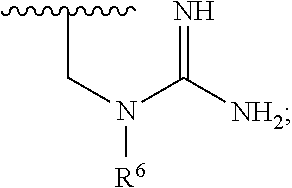
Indole, azaindole and related heterocyclic ureido and thioureido piperazine derivatives
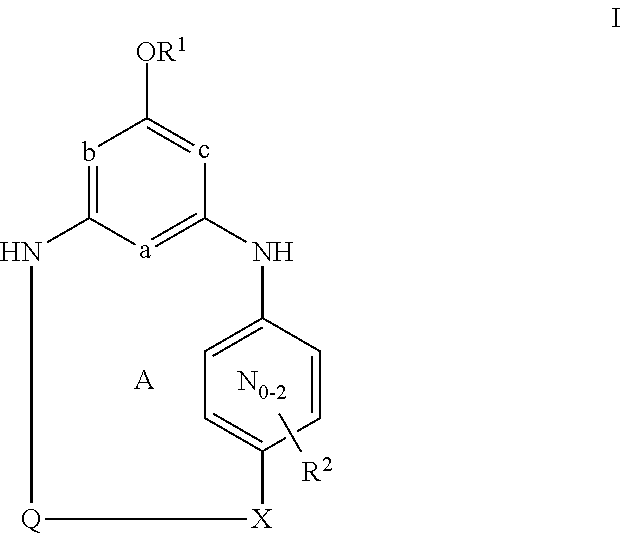
This invention provides compounds having drug and bio-affecting properties, their pharmaceutical compositions and method of use. In particular, the invention is concerned with ureido and thioureido piperazine derivatives of Formula I. These compounds possess unique antiviral activity, whether used alone or in combination with other antivirals, antiinfectives, immunomodulators or HIV entry inhibitors. More particularly, the present invention relates to the treatment of HIV and AIDS. The compounds of Formula I are wherein: Y is O or S; Q is selected from the group consisting of m is 2; A is NR13R14; and —W— is
Owner:REGUEIRO REN ALICIA +3
Use of trem-1 inhibitors for treatment, elimination and eradication of hiv-1 infection
ActiveUS20180185372A1Reduce inflammationMinimize abilityOrganic active ingredientsPeptide/protein ingredientsChikungunyaVirus inhibitors
Compounds, compositions, and methods of treatment and prevention of HIV, including HIV-1 and HIV-2, Dengue, and Chikungunya infection are disclosed. The compounds are TREM-1 inhibitors. Combinations of these TREM-1 inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, JAK inhibitors, macrophage depleting agents, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV, Dengue, or Chikungunya virus in an infected patient.
Owner:EMORY UNIVERSITY
Screening for hepatitis c virus entry inhibitors
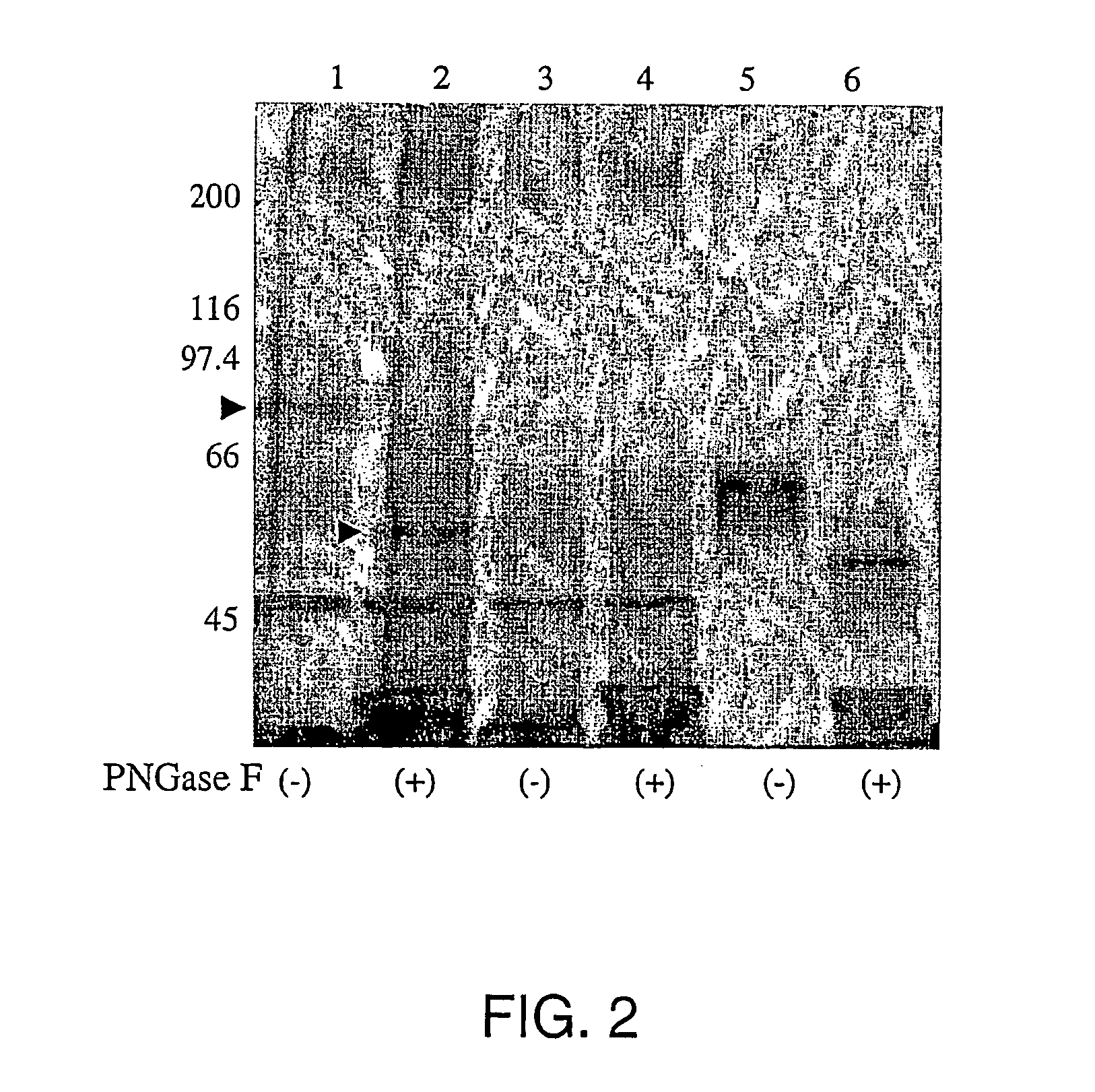
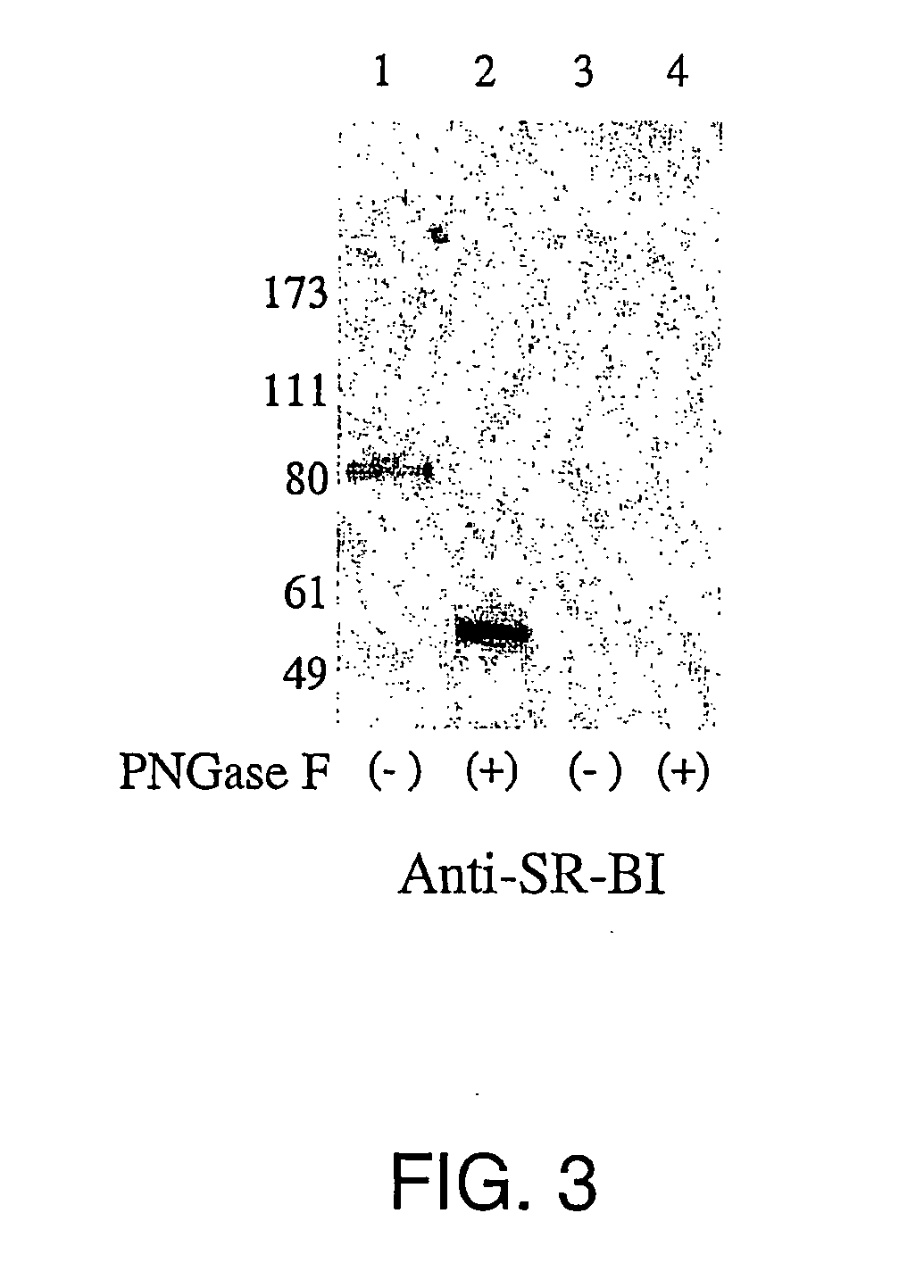
InactiveUS20050019751A1Inhibit bindingDecreasing functional surface expressionCompound screeningApoptosis detectionScavenger receptorProphylactic treatment
The present invention features methods of screening for compounds that inhibit HCV binding to a cell, methods of inhibiting IICV entry into a cell, and methods of actively or prophylactically treating against an IICV infection. The different methods are based on the identification of the scavenger receptor class B type I as a target site for HCV E2 binding to a cell.
Owner:IST DI RICERCHE DI BIOLOGIA MOLECOLARE P ANGELETTI
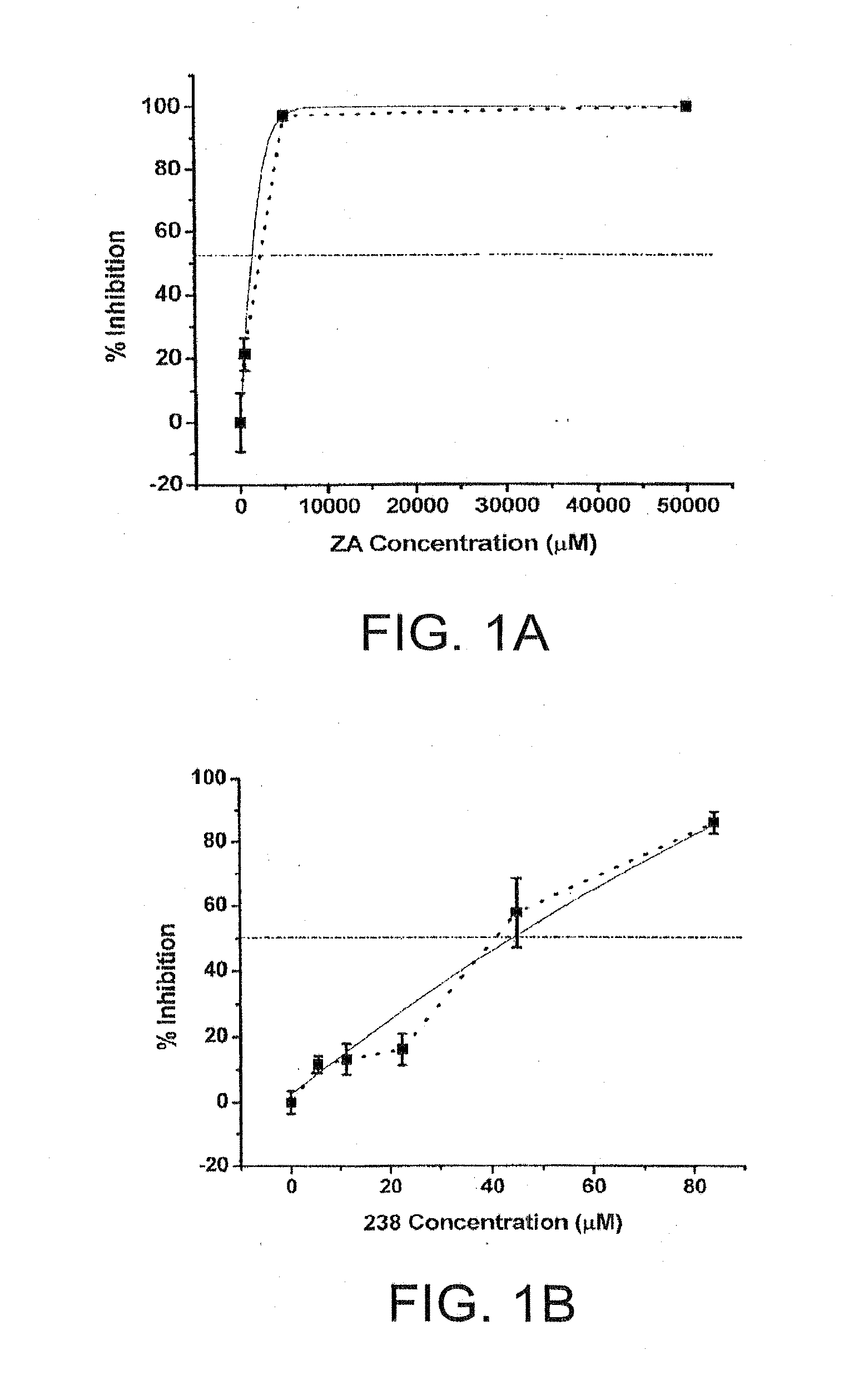
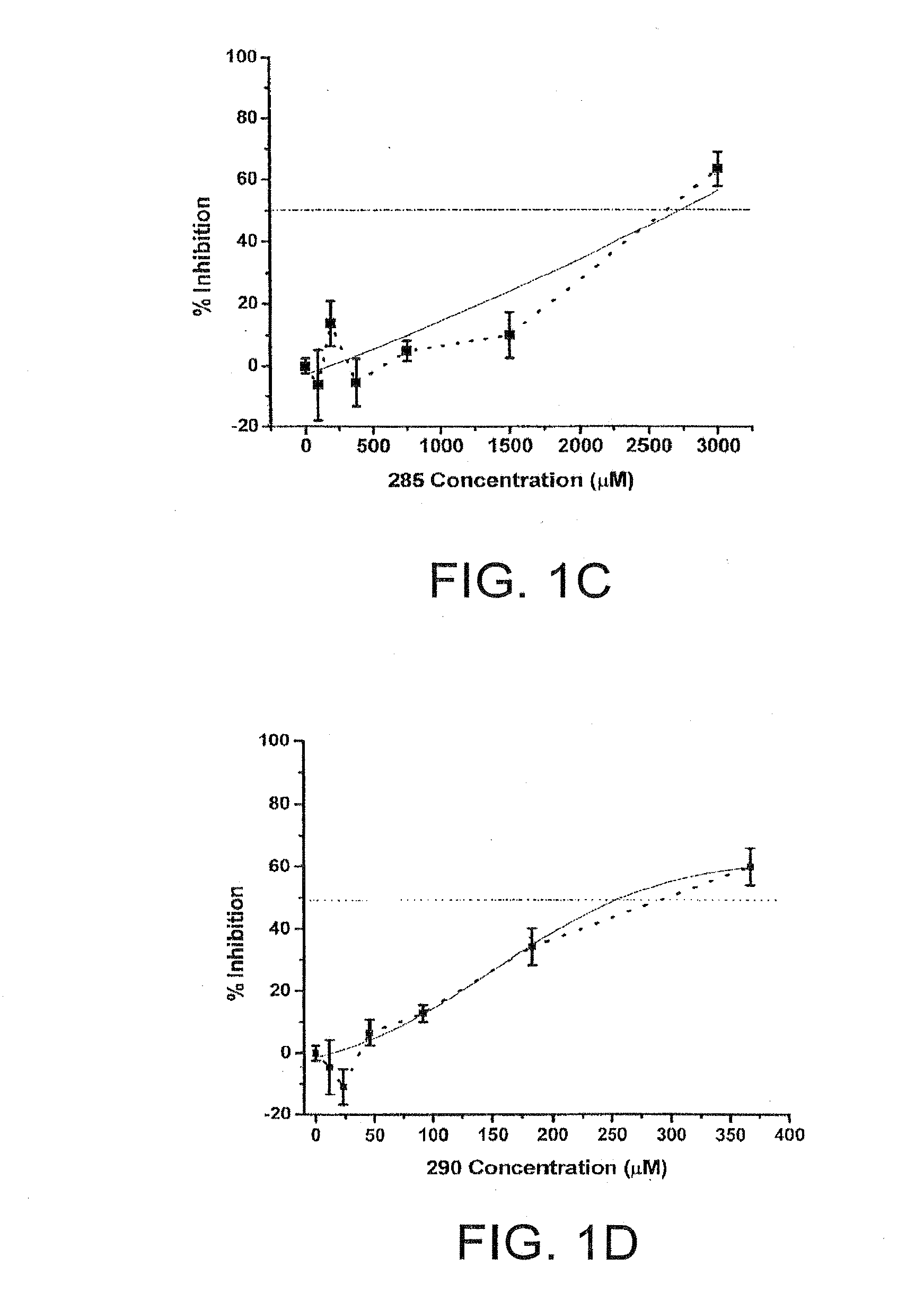
Anti-viral properties of zosteric acid and related molecules
The invention relates chemical compound entry inhibitors and methods of determining such inhibitors that interact with regions of viruses, such as the dengue virus, as candidates for in vivo anti-viral compounds.
Owner:FLORIDA GULF COAST UNIVERSITY
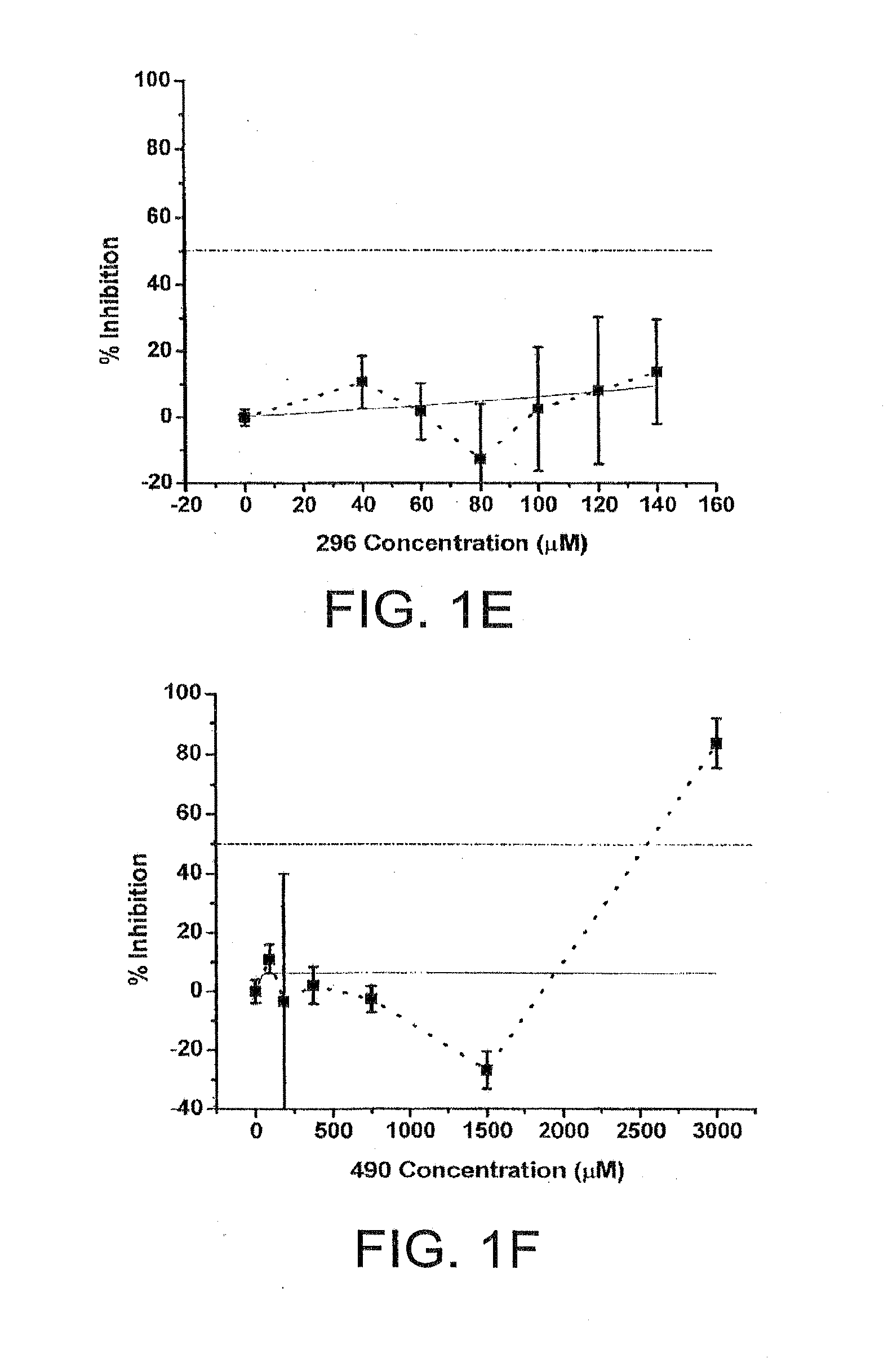
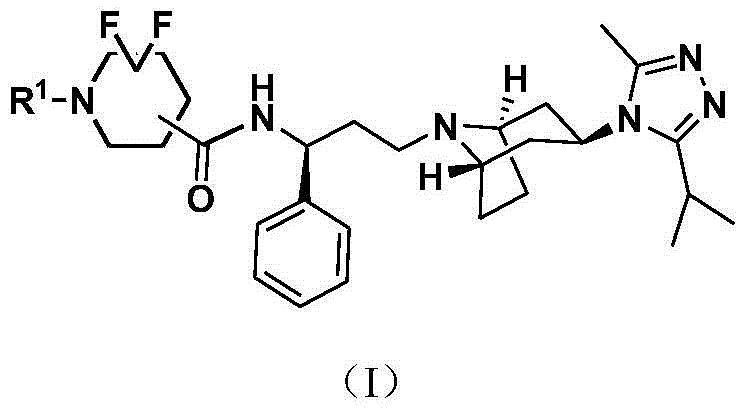
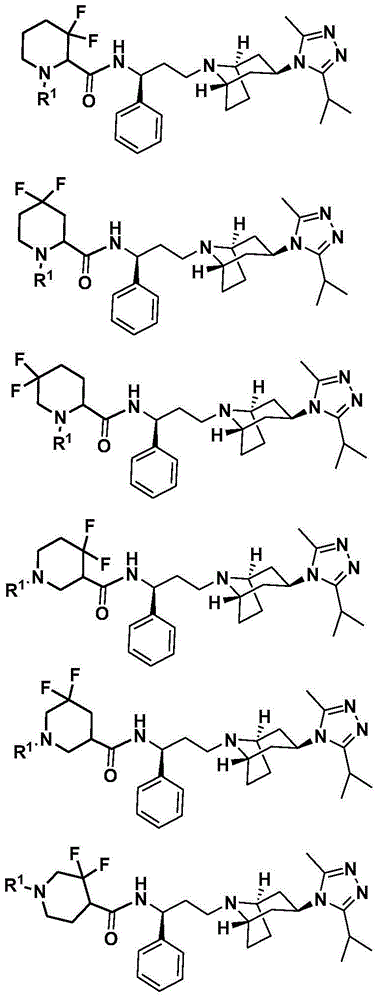
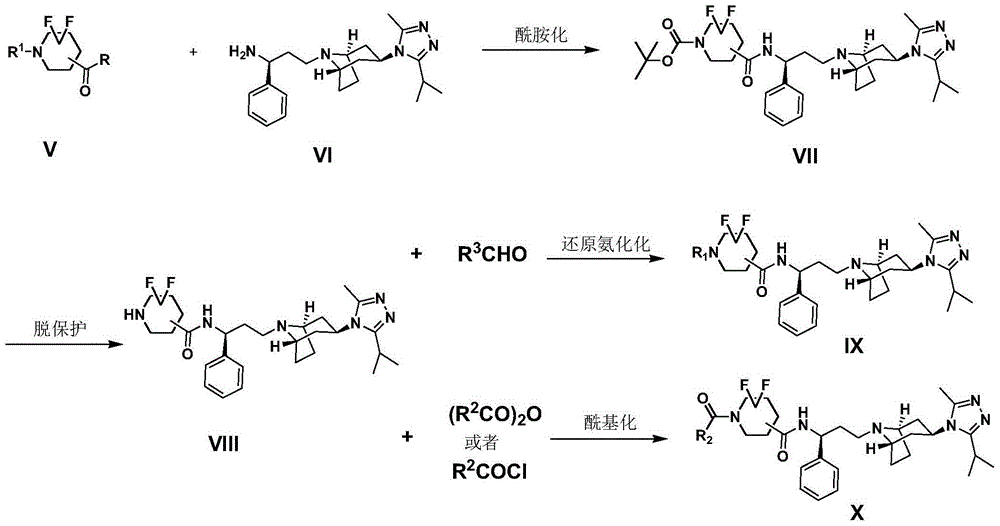
Difluoro methylene piperidine carboxamide derivative as well as preparation method and application thereof
ActiveCN104387379AStrong CCR5 antagonistic activityOrganic chemistryAntiviralsHIV receptorChemokine receptor CCR5
The invention relates to the field of compound synthesis, and mainly relates to a difluoro methylene piperidine carboxamide derivative (I), a medicament composition containing the difluoro methylene piperidine carboxamide derivative (I), and a preparation method and application thereof. The definition of R<1> is shown in the specification. Proved by pharmacodynamic tests, a compound disclosed by the invention has a CCR5 antagonistic action, can be used as an entry inhibitor of HIV virus, and can also be developed to be anti-AIDS medicines.
Owner:SHANGHAI AQ BIOPHARMA CO LTD
Optimized dengue virus entry inhibitory peptide (DN81)
Owner:FLORIDA GULF COAST UNIV BOARD OF TRUSTEES +1
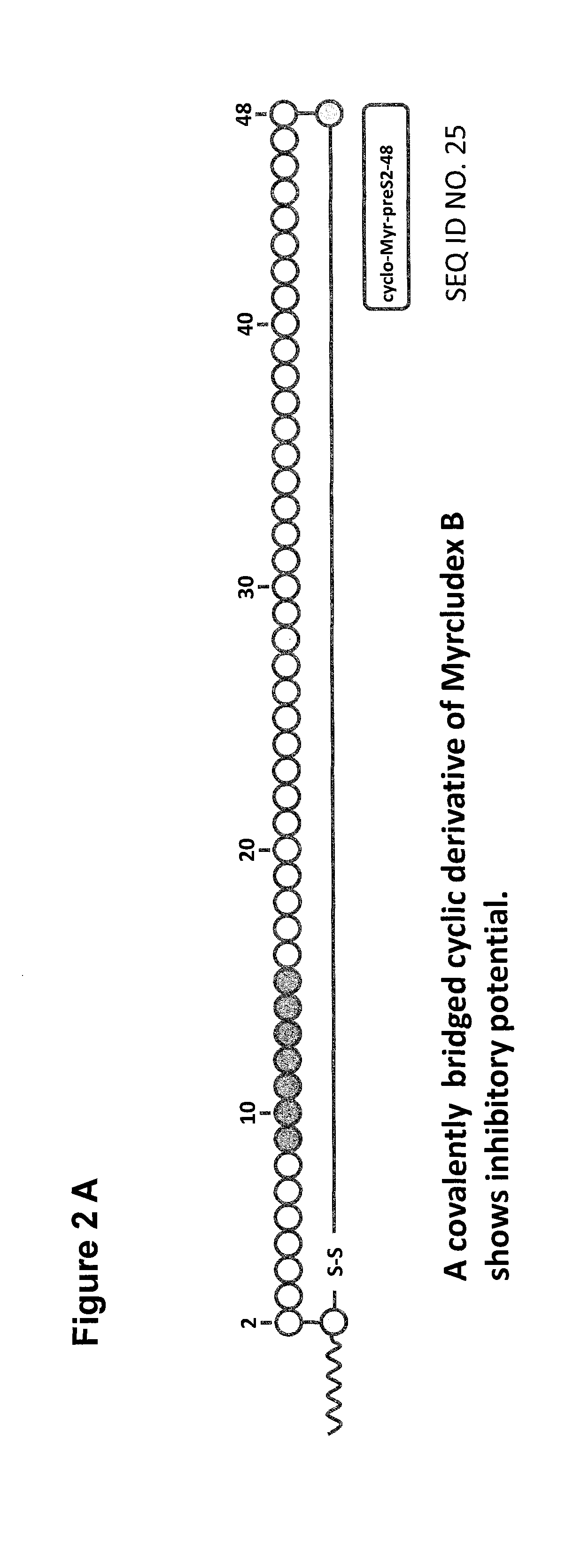
Cyclic ntcp-targeting peptides and their uses as entry inhibitors
ActiveUS20180354993A1Inhibit receptor functionSufficient inhibitionPeptide-nucleic acidsIn-vivo radioactive preparationsCyclic peptideDisease
The present invention relates to cyclic NTCP targeting peptides which are preS-derived peptides of hepatitis B virus (HBV). The present invention further relates to pharmaceutical compositions comprising at least one cyclic peptide. The present invention further relates to medical uses of said cyclic peptides and the pharmaceutical compositions, such as in the diagnosis, prevention and / or treatment of a liver disease or condition, and / or in the inhibition of HBV and / or HDV infection. The present invention further relates to methods of diagnosis, prevention and / or treatment of a liver disease or condition and / or the inhibition of HBV and / or HDV infection.
Owner:UNIVERSITY OF HEIDELBERG
Methods for Treating HCV
The present invention features interferon-free therapies for the treatment of HCV. The therapies comprise administering 2R,6S,13aS,14aR,16aS,Z)-N-(cyclopropylsulfonyl)-6-(5-methylpyrazine-2-carboxamido)-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,5,6,7,8,9,10,11,13a,14,14a,15,16,16a-exadecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a-carboxamide (Compound I), or a pharmaceutically acceptable salt thereof, and another anti-HCV agent. Preferably, the therapies are both interferon- and ribavirin-free. The other anti-HCV agent can be a HCV polymerase inhibitor, an HCV NS5A inhibitor, an HCV entry inhibitor, a cyclophilin inhibitor, or an internal ribosome entry site inhibitor. Preferably, the other anti-HCV agent is an HCV polymerase inhibitor. Also preferably, the other anti-HCV agent is an HCV NS5A inhibitor. Also preferably, the other anti-HCV agent is administered concurrently with Compound I or a pharmaceutically acceptable salt thereof. In another example, the other anti-HCV agent is administered sequentially with Compound I or a pharmaceutically acceptable salt thereof. In still another embodiment, Compound I (or a pharmaceutically acceptable salt thereof) is co-administered with two or more other anti-HCV agents. For instance, Compound I (or a pharmaceutically acceptable salt thereof) can be co-administered with an HCV polymerase inhibitor and an HCV NS5A inhibitor. For another instance, Compound I (or a pharmaceutically acceptable salt thereof) can be co-administered with two different HCV polymerase inhibitors (e.g., one is a nucleoside polymerase inhibitor and the other is a non-nucleoside polymerase inhibitor; or both are nucleoside polymerase inhibitors; or both are non-nucleoside polymerase inhibitor). In yet another example, Compound I (or a pharmaceutically acceptable salt thereof) is co-administered with another HCV protease inhibitor and an HCV polymerase inhibitor. In still another example, Compound I (or a pharmaceutically acceptable salt thereof) is administered with two different HCV NS5A inhibitors.
Owner:ABBVIE INC
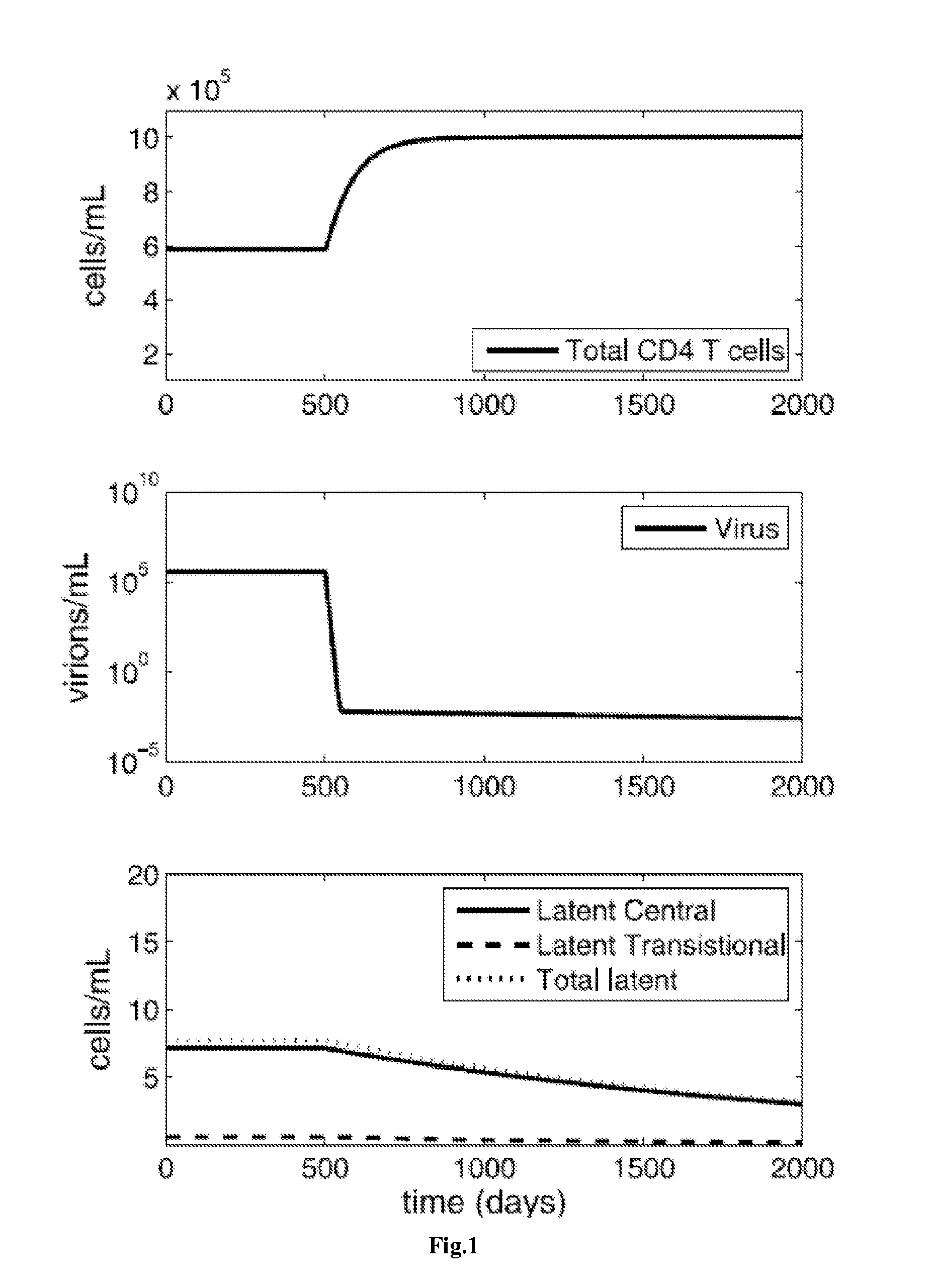
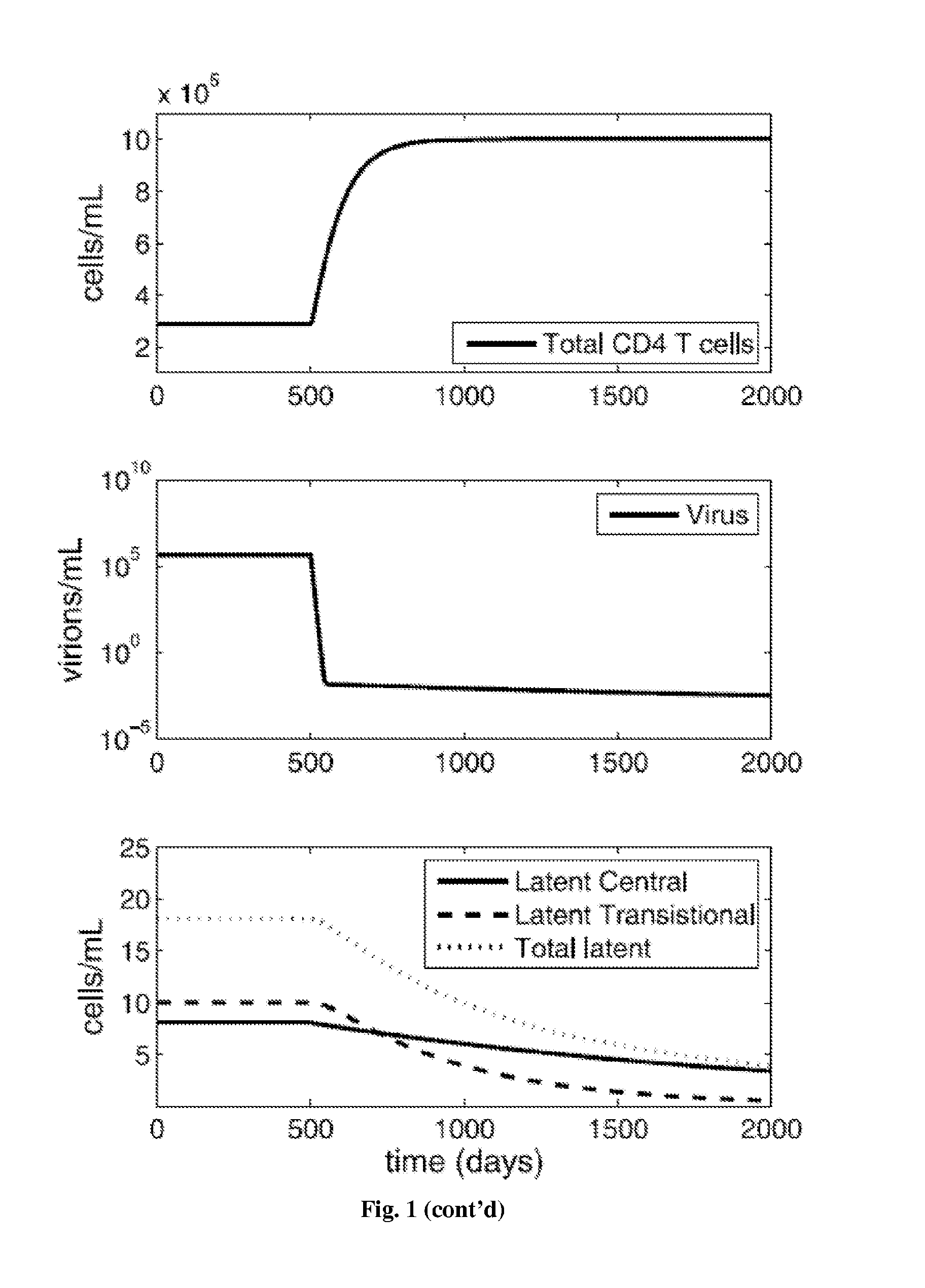
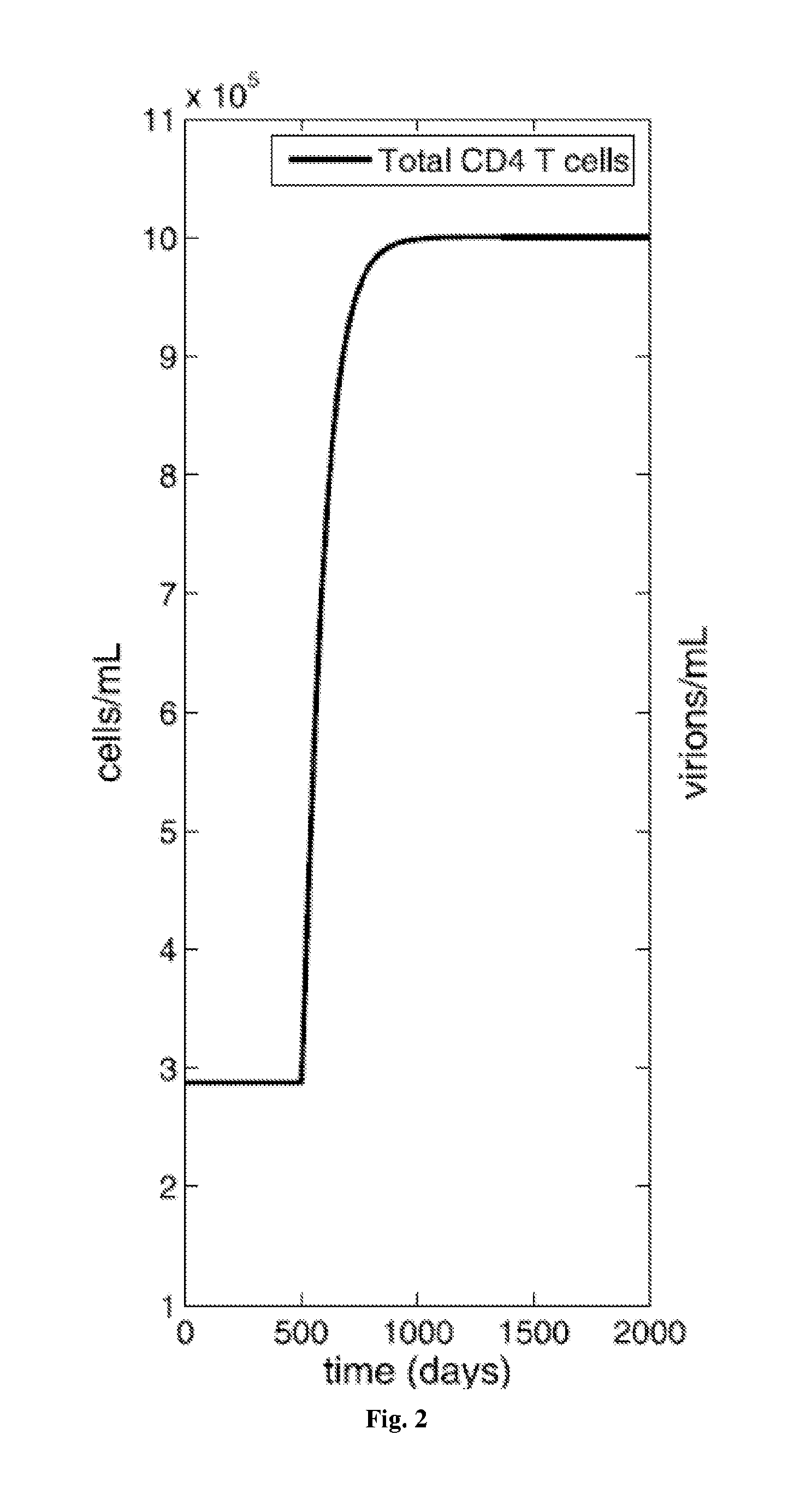
Treatment of latent HIV infection
InactiveUS20150320893A1EliminateEncourage viral productionBiocideAntiviralsReverse transcriptaseProstaglandin analog
Methods for treating HIV positive patients, and purging and eradicating latent HIV virus from a patient's system, are disclosed. The bulk of viral load is eradicated using conventional antiretroviral (ARV) therapy. Compounds that encourage viral production in the latent cells are then administered, preferably without activating those cells, while maintaining the ARV therapy. The administration of compounds that encourage viral production in latent cells is cycled, and after around 7-10 cycles, the methods can virtually eliminate latent HIV in the patient. Ideally, the ARV regimen includes at least one integrase inhibitor, at least one entry inhibitor, such as a CCR5 antagonist, and at least one, and preferably two, reverse transcriptase inhibitors. The compounds that encourage viral production in latent cells ideally include a combination of prostratin or a prostratin analog and an HDAC inhibitor, such as butyrate, valproate, or SAHA.
Owner:VOLPE JOSEPH M
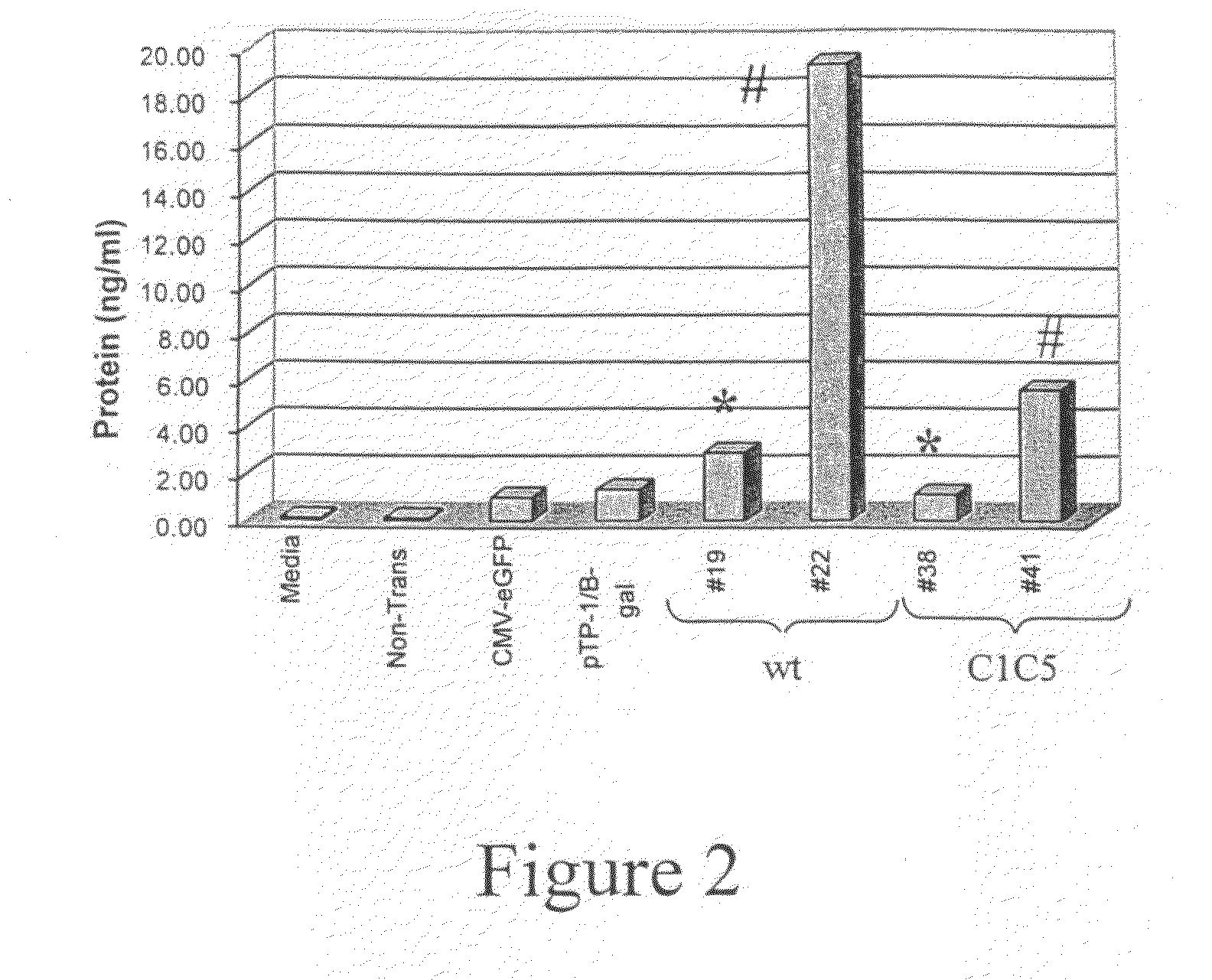
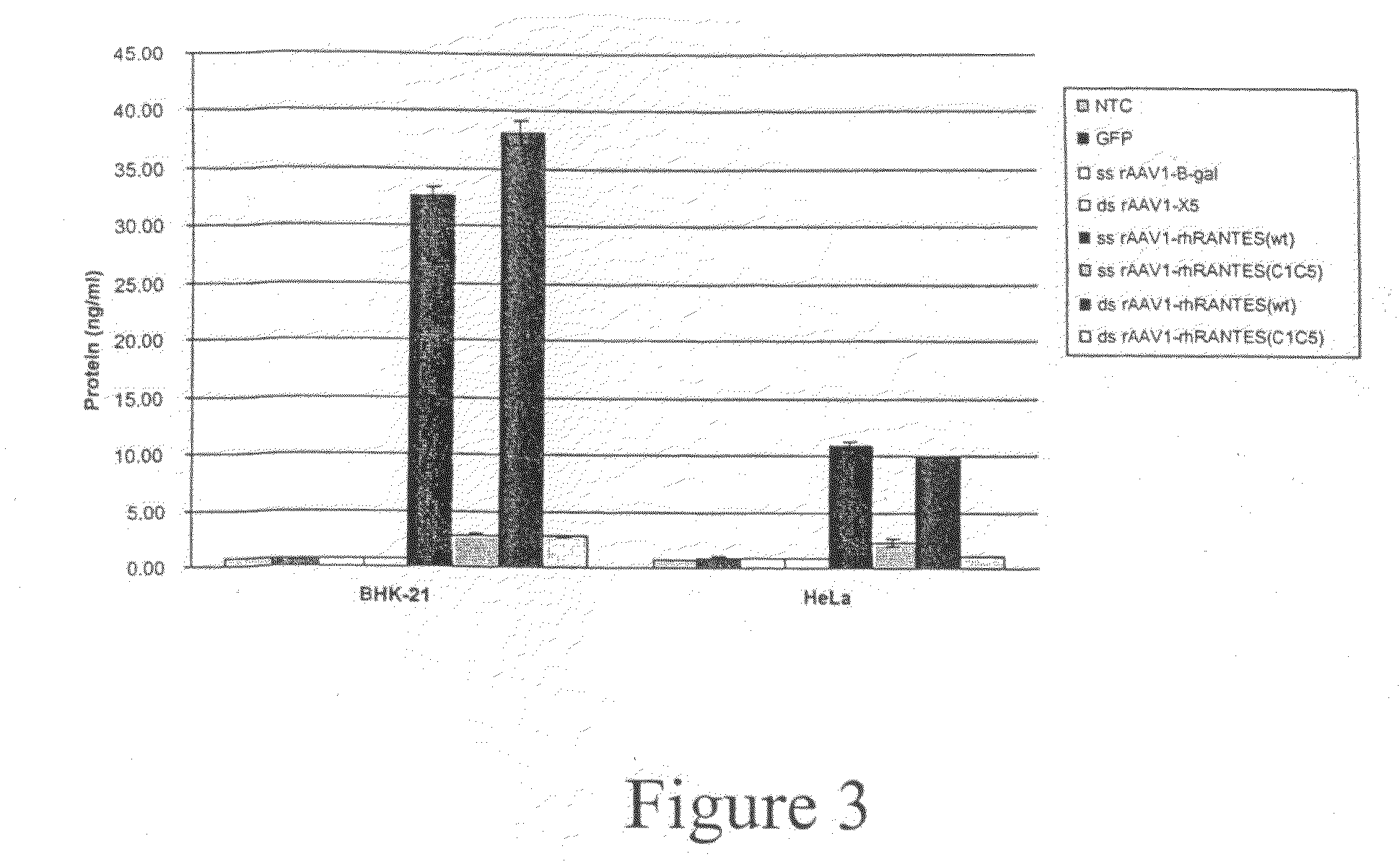
Expression of virus entry inhibitors and recombinant AAV therefor
InactiveUS20090169513A1Enhanced secretion and stabilityEase of administration and handlingBiocideSugar derivativesGene deliveryMammal
The present invention relates generally to the use of recombinant adeno-associated viruses (rAAV) for gene delivery and more specifically to the use of rAAV to deliver genes encoding human immunodeficiency virus entry inhibitors to target cells in mammals.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Inhibitors of hiv-1 entry and methods of use thereof
ActiveUS20170233335A1Avoid transmissionInhibit progressOrganic active ingredientsVirus peptidesEpitopeMicrobacterium
The disclosure provides compositions and methods for sensitizing primary HIV-1, including transmitted / founder viruses, to neutralization by monoclonal antibodies, e.g., those directed against CD4-induced (CD4i) epitopes and the V3 region. In certain embodiments, the disclosure relates to the use of small molecules as microbicides to inhibit HIV-1 infection directly and to sensitize primary HIV-1 to neutralization by readily elicited antibodies.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Low molecular weight carboxyl-reduced derivatives of fucosylated glycosaminoglycans and preparation method and applications of low molecular weight carboxyl-reduced derivatives
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com