Low molecular weight glycosylated chondroitin sulfate and its purpose in preparation of anti-HIV-1 medicament
A chondroitin sulfate and low-molecular-weight sugar technology, which is used in medical preparations, antiviral agents, and pharmaceutical formulations containing active ingredients, can solve problems such as significant toxic and side effects, drug resistance, and non-response to treatment. Separation and purification, stable depolymerization speed, and the effect of avoiding drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] [Example 1] Preparation of low molecular weight glycosylated chondroitin sulfate (LGC)
[0067] 1.1 Materials:
[0068] Plum ginseng (Thelenota ananas Jaeger), commercially available, gutted and dried body wall;
[0069] H 2 O 2 , CH 3 COONa 3H 2 O, NaCl, NaOH, Cu(CH 3 COO) 2 ·H 2 The reagents used in O etc. are all commercially available analytically pure reagents.
[0070]1.2 Method:
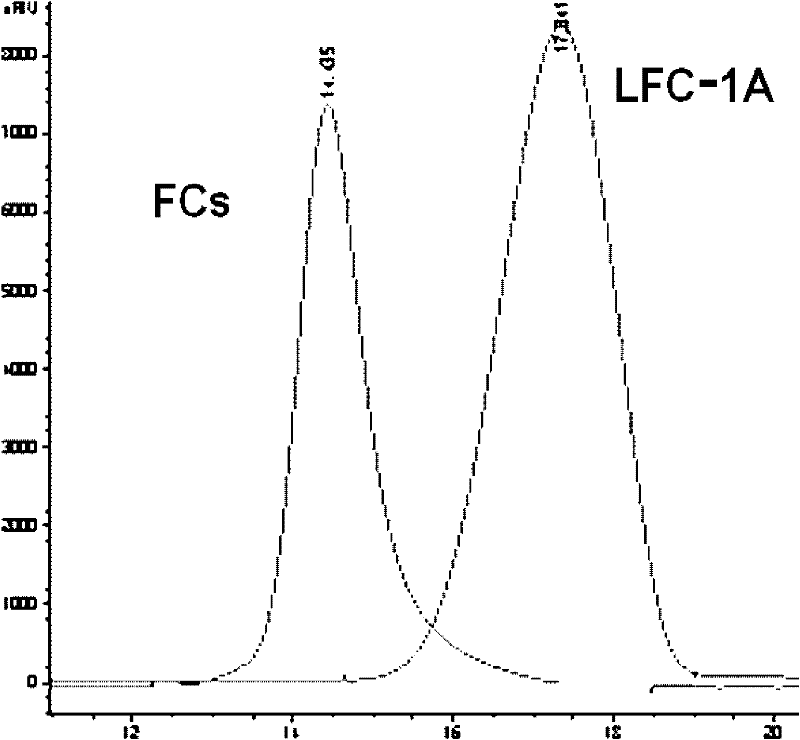
[0071] (1) Preparation of Glycosylated Chondroitin Sulfate (GCs): Take the dried body wall of Echinoderm Prunus chinensis, and prepare GCs according to the literature method (J Biol Chem, 1991, 266(21): 13530-6), with a yield of 0.75% , purity 98% (HPGPC, area normalization method), weight average molecular weight (Mw), 65,820.
[0072] (2) Preparation of low-molecular-weight glycosylated chondroitin sulfate (LGC): 5.0 g of GCs obtained in step (1) was added to a round-bottomed flask, 180 ml of distilled water was added to dissolve it, the temperature was kept in a water bath ...
Embodiment 2
[0090] [Example 2] Preparation of a series of molecular weight LGC-1
[0091] 2.1 Materials:
[0092] The GCs derived from Plum ginseng were prepared by the method described in Example 1.
[0093] H 2 O 2 , CH 3 COONa 3H 2 O, NaCl, NaOH, Cu(CH 3 COO) 2 ·H 2 The reagents used in O etc. are all commercially available analytically pure reagents.
[0094] 2.2 Method:
[0095] (1) Preparation of LGC-1 samples with a series of molecular weights: Four GCs derived from Plum ginseng, 5 g each, were depolymerized by the method described in step (2) of Example 1, but the time points for terminating the reaction were 90, 120, 360, and 420 min, respectively. . The LGC-1 products thus prepared are respectively numbered: LGC-1B, LGC-1C, LGC-1D and LGC-1E. The yields of the depolymerization reactions were all greater than 70%.
[0096] (2) LGC-1 product detection: HPGPC detection of molecular weight and distribution; conductivity detection-OSO 3 - / -COO - Molar ratio; optical r...
Embodiment 3
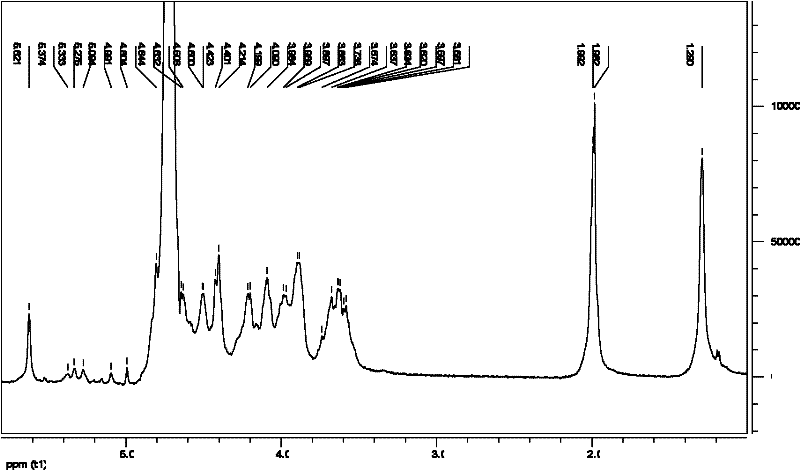
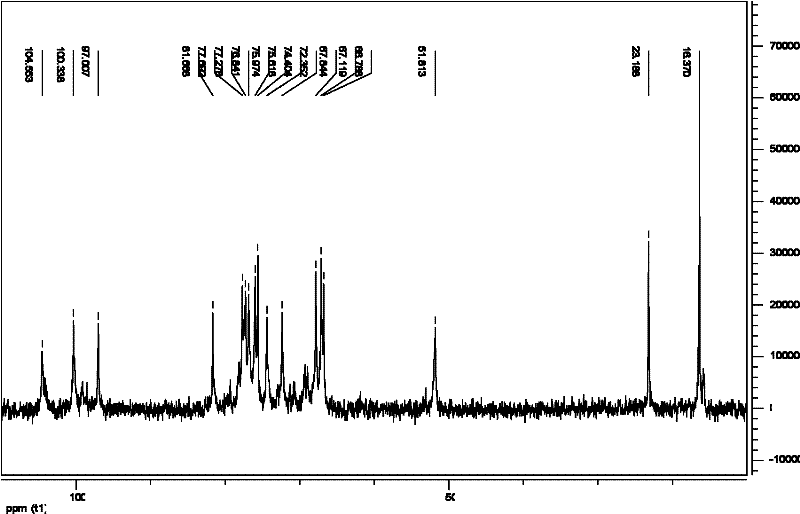
[0103] [Example 3] Terminal reductive amination of LGC-1A
[0104] 3.1 Materials:
[0105] LGC-1A: Example 1 Preparation of depolymerized GCs product.
[0106] Tyramine hydrochloride and sodium cyanoborohydride: both are commercially available analytical reagents.
[0107] 3.2 Method:
[0108] (1) Terminal reductive amination of LGC-1A: 1000 mg of GCs depolymerization product LGC-1A was dissolved in 35 ml of 0.2 mM phosphate buffer (PBS, pH 8.0), and an excess of 800 mg of tyramide and 300 mg of cyanoboron were added during stirring. Sodium hydride was reacted in a constant temperature water bath at 35°C for about 100hr. After the reaction is completed, add 105 ml of 95% ethanol, and centrifuge to obtain a precipitate. After the obtained precipitate is washed twice with 30 ml of 95% ethanol, the obtained precipitate is redissolved with 35 ml of 0.1% NaCl, centrifuged to remove insoluble matter, and the supernatant is placed on Seph adex. TM G-100 column, eluted with 0.1% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com