Indole, azaindole and related heterocyclic N-substituted piperazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 4
LC / MS: (ES+) m / z (M+H)+=493, 495; HPLC Rt=1.128.
A mixture of Example 4 (50 mg, 0.101 mmol), imidazole (69 mg, 1.01 mmol) cesium carbonate (66 mg, 0.203 mmol) and copper bromide (30 mg, 0.212 mmol) was heated at 145° C. for 4 h. The reaction mixture was then cooled to r.t., diluted with MeOH (2 ml) and filtered. The residue was further washed with 3×2 ml MeOH. The filtrate was evaporated in vacuo to give the crude product, which was purified by preparative TLC (10% MeOH / CH2Cl2) to give Example 5; LC / MS: (ES+) m / z (M+H)+=481; HPLC Rt=0.867.
example 6
Example 6 was prepared in the same manner as Example 5.
LC / MS: (ES+) m / z (M+H)+=480, 495; HPLC Rt=1.233.
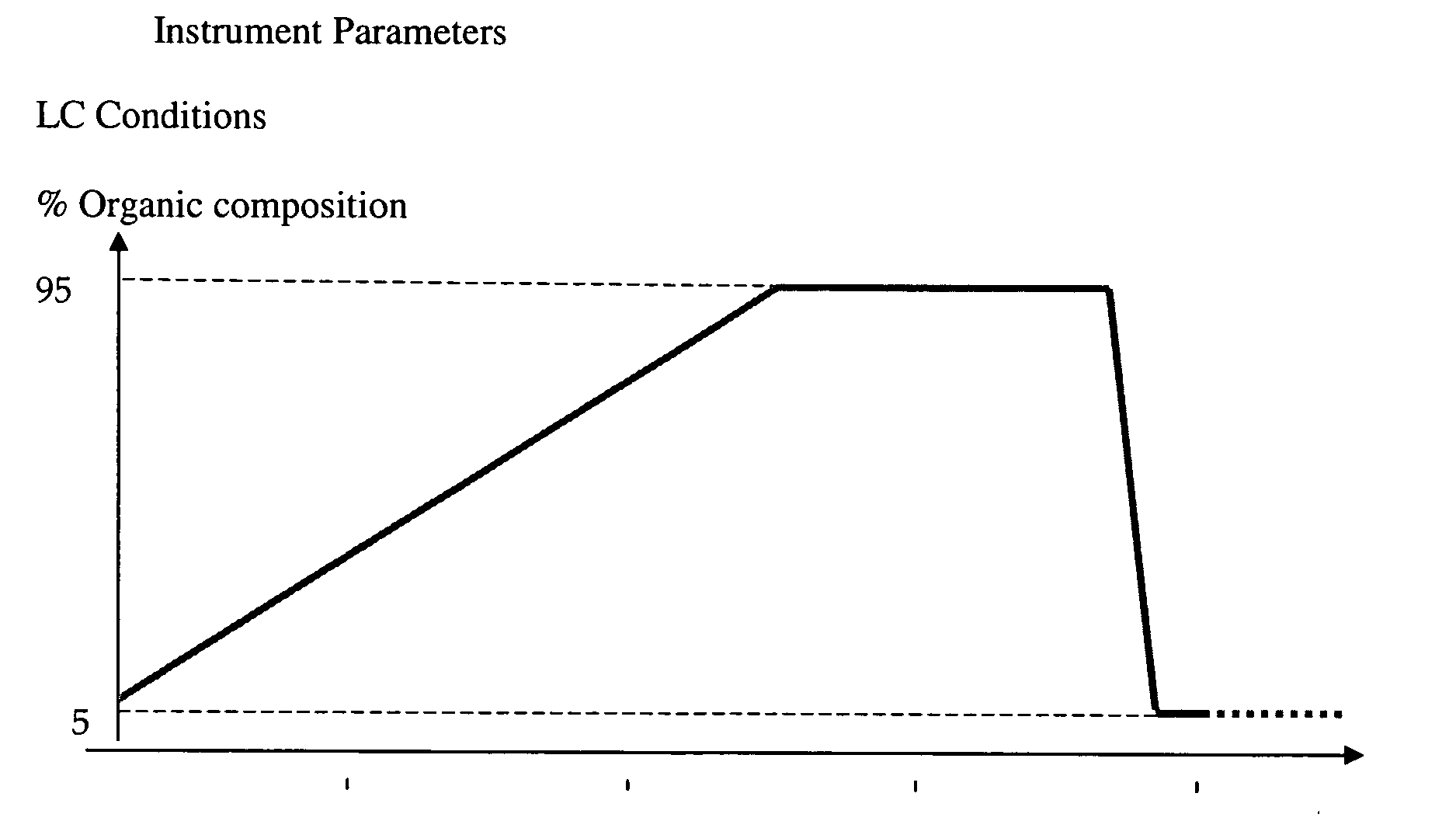
The following HPLC conditions for the LCMS were used for Example 7, Example 8 and Example 9: Column: G; Gradient Time=3 min; Flow rate=4 ml / min.
Preparation of Example 7:
To a mixture of 4ab (crude, about 1.94 mmol), DEPBT (1.161 g, 3.88 mmol), intermediate 2 (952 mg, 2.91 mmol) in DMF (5 ml) was added N,N-diisopropylethylamine (3.0 ml, 17 mmol). The reaction mixture was stirred at room temperature for 16 hours. The reaction mixture was then diluted with MeOH (6 ml) and filtered. The filtrate was purified by preparative reverse phase HPLC using the method: Start % B=20, Final % B=60, Gradient time=15 min, Flow Rate=40 ml / min, Column: XTERRA C18 5 μm 30×50 mm, Fraction Collection: 6.169-6.762 min. 1H NMR: (DMSO-d6) 13.71 (s, 1H), 8.50 (d, J=3.0, 1H), 8.27 (s, 1H), 8.23 (d, J=8.5, 1H), 8.06 (d, J=6.0, 1H), 7.97 (d, J=8.0, 1H), 7.82 (app t, J=7.5, 1H), 7.69 (d, J=7.5, 1H), 7.5...
example 43
A mixture of intermediate 4ab (0.671 g, 2.7 mmol), intermediate 2 (0.869 g, 4.1 mmol), EDC (0.928 g, 4.8 mmol), dimethylaminopyridine (0.618 g, 5.1 mmol) and N-methylmorpholine (2.4 ml, 21.6 mmol) in DMF (20 ml) was stirred at room temperature for 17 hr. The reaction mixture was then quenched with 1N HCl and extracted with ethyl acetate (6 times). The combined organic extracts were evaporated in vacuo and purified by flash columatography (0%→5% MeOH / CH2Cl2) to provide Example 43 as a dark solid; 1H NMR (CDCl3) δ 9.64 (b s, 1H), 8.15 (d, J=5.5, 1H), 8.11 (d, J=8.0, 1H), 8.05 (d, J=3.0, 1H), 7.79 (d, J=8.0, 1H), 7.65 (app t, J=9.0, 1H), 7.57 (d, J=8.5, 2H), 7.32 (d, J=6.0, 1H), 6.74 (d, J=8.5, 1H), 4.05 (s, 3H, overlapping with m), 4.05-4.00 (m, 2H), 3.79 (b s, 2H), 3.56 (b s, 2H), 3.48 (b s, 2H); LC / MS (ES+) m / z (M+H)+=440, HPLC Rt=0.993.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com