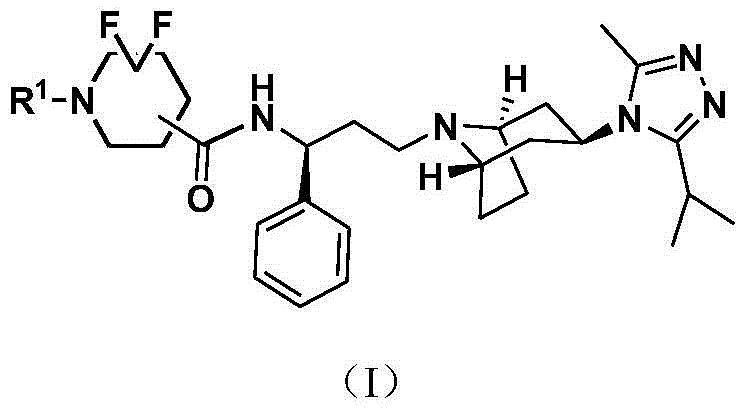
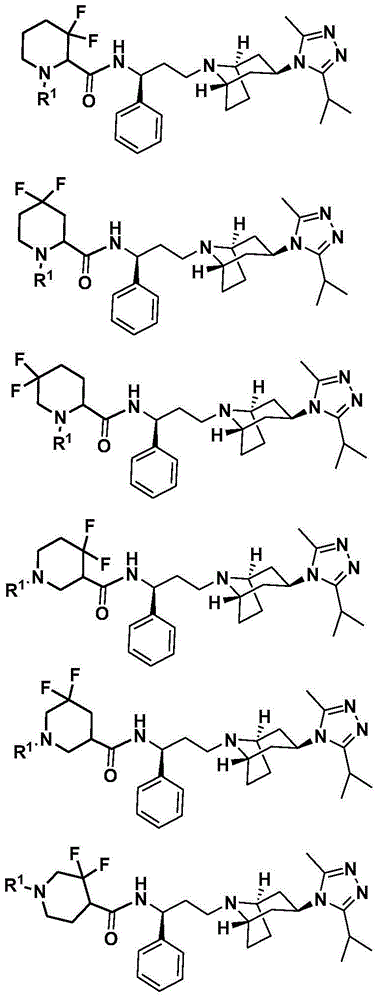
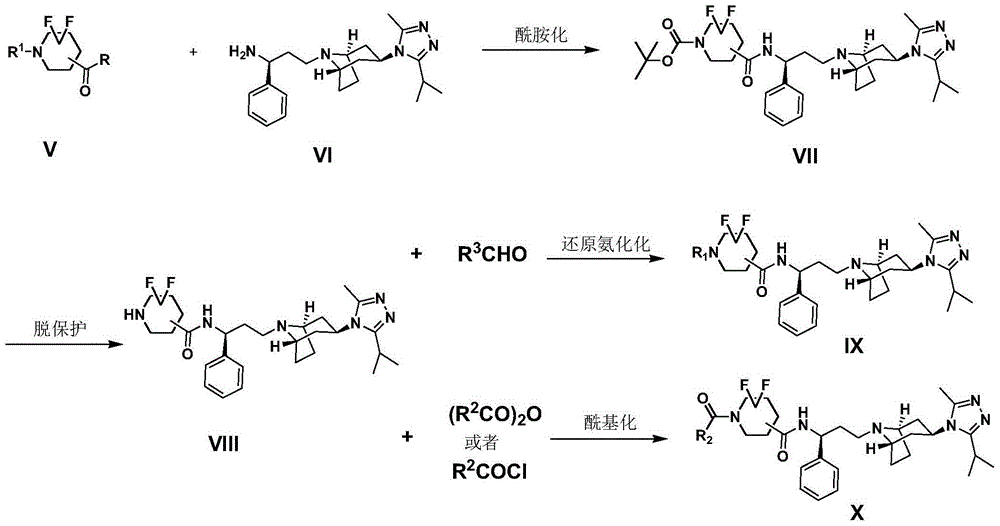
Difluoro methylene piperidine carboxamide derivative as well as preparation method and application thereof
A technology of difluoromethylene piperidine carboxamide and derivatives, which is applied in the field of compound synthesis, can solve the problems of toxicity, cost, adverse reactions and the like of anti-HIV drugs, and achieve the effect of strong CCR5 antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Compound 3:
[0046]
[0047] Preparation method: compound 1-a (0.1g, 0.38mmol, 1.0eq), compound 2 (0.139g, 0.38mmol, 1.0eq), EDCI (0.11g, 1.5eq), HOBT (0.077g, 1.5eq) were dissolved in 10ml dry THF, N 2 Stir overnight at room temperature under protection. TLC showed that the reaction of the raw materials was complete. Concentrate directly, and the mixture was directly separated and purified by silica gel column chromatography to obtain the product compound 3.
[0048] 1 H NMR (400MHz, CDCl3) δ7.38~7.27(m,5H),5.13~5.09(br,1H),4.78(br,1H),4.28~4.27(br,2H),3.38(br,2H), 3.01~2.97(br,2H),2.51(s,3H),2.42~2.38(m,2H),2.28~1.86(m,11H),1.64~1.62(br,4H),1.49~1.41(m,15H ).MS-ESI(+):cal.614,found:615(M+H).
Embodiment 2
[0050] Preparation of Compound 4:
[0051]
[0052] Preparation method: using compound 1-b and compound 2 as raw materials, the preparation method is the same as in Example 1.
[0053] 1 H NMR (400MHz, CDCl3) δ7.38~7.27(m,5H), 5.13~5.09(br,1H), 4.28~4.27(br,3H), 3.41~3.30(br,4H), 3.19(br,1H) ),3.01(br,1H).2.52~2.48(m,6H),2.23~2.14(m,5H),2.05~1.96(m,7H),1.46(br,9H),1.39~1.38(dd,6H ).MS-ESI(+):cal.614,found:615(M+H).
Embodiment 3
[0055] Preparation of Compound 6:
[0056]
[0057] Preparation method: using compound 1-c and compound 2 as raw materials, the preparation method is the same as in Example 1,
[0058] 1 H NMR (400MHz, CDCl3) δ7.65(br,1H),7.45~7.35(m,2H),7.29~7.24(m,3H),5.13~5.09(br,1H),4.16~4.13(m,3H ), 3.41~3.38(br,2H), 2.99~2.97(br,3H), 2.50~2.45(dd,3H), 2.44~1.99(m,11H), 1.67~1.63(br,4H), 1.47~ 1.44(dd,9H),1.39~1.38(dd,6H).MS-ESI(+):cal.614,found:615(M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com