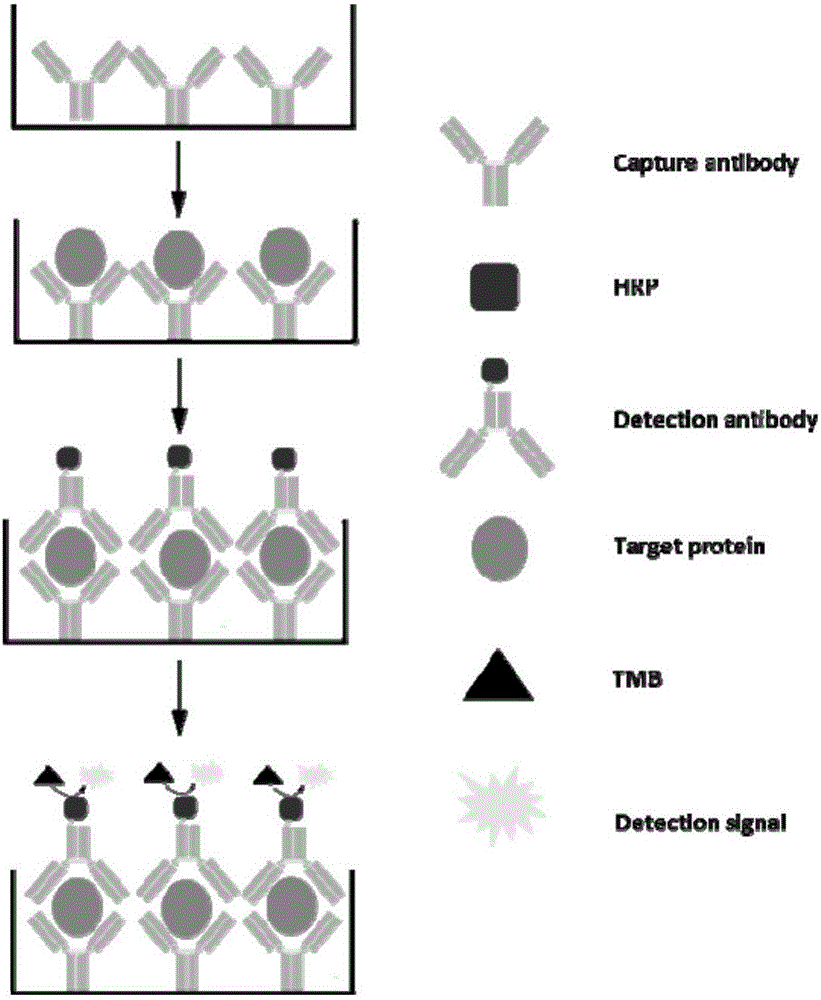
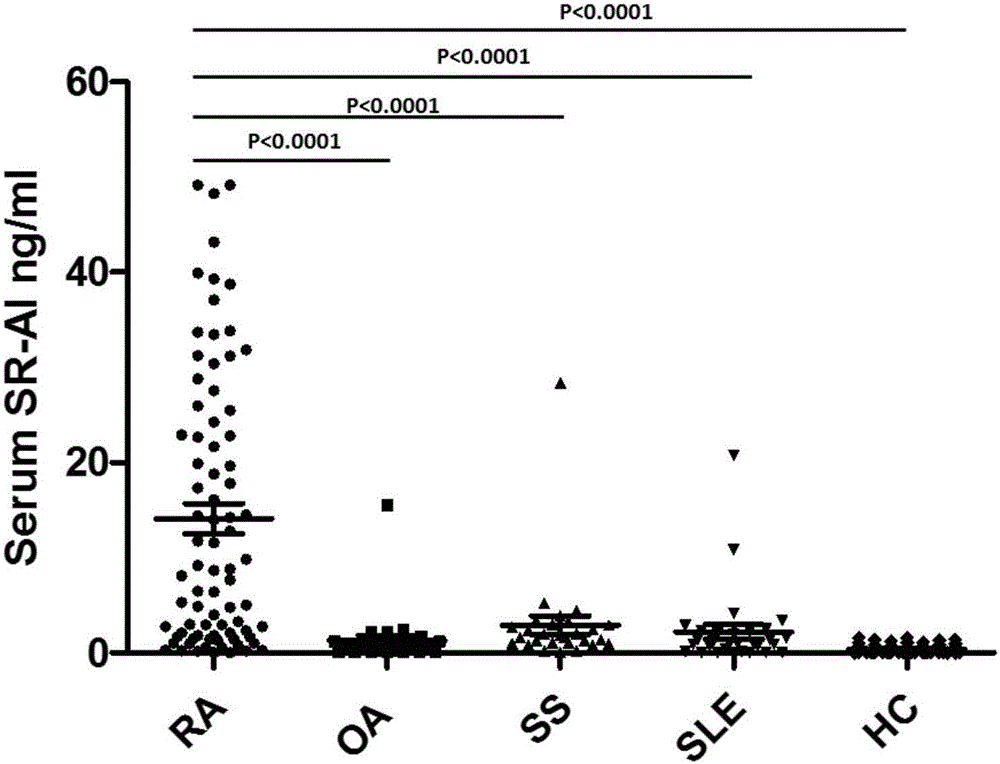
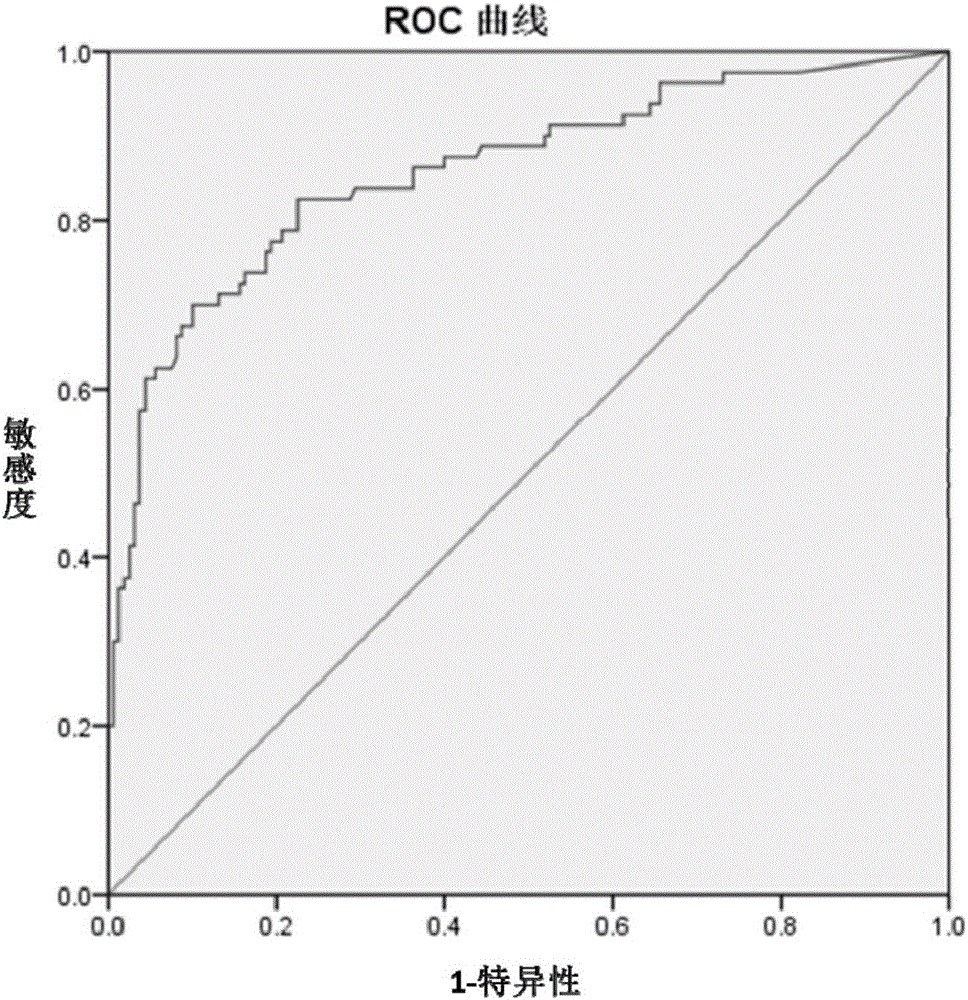
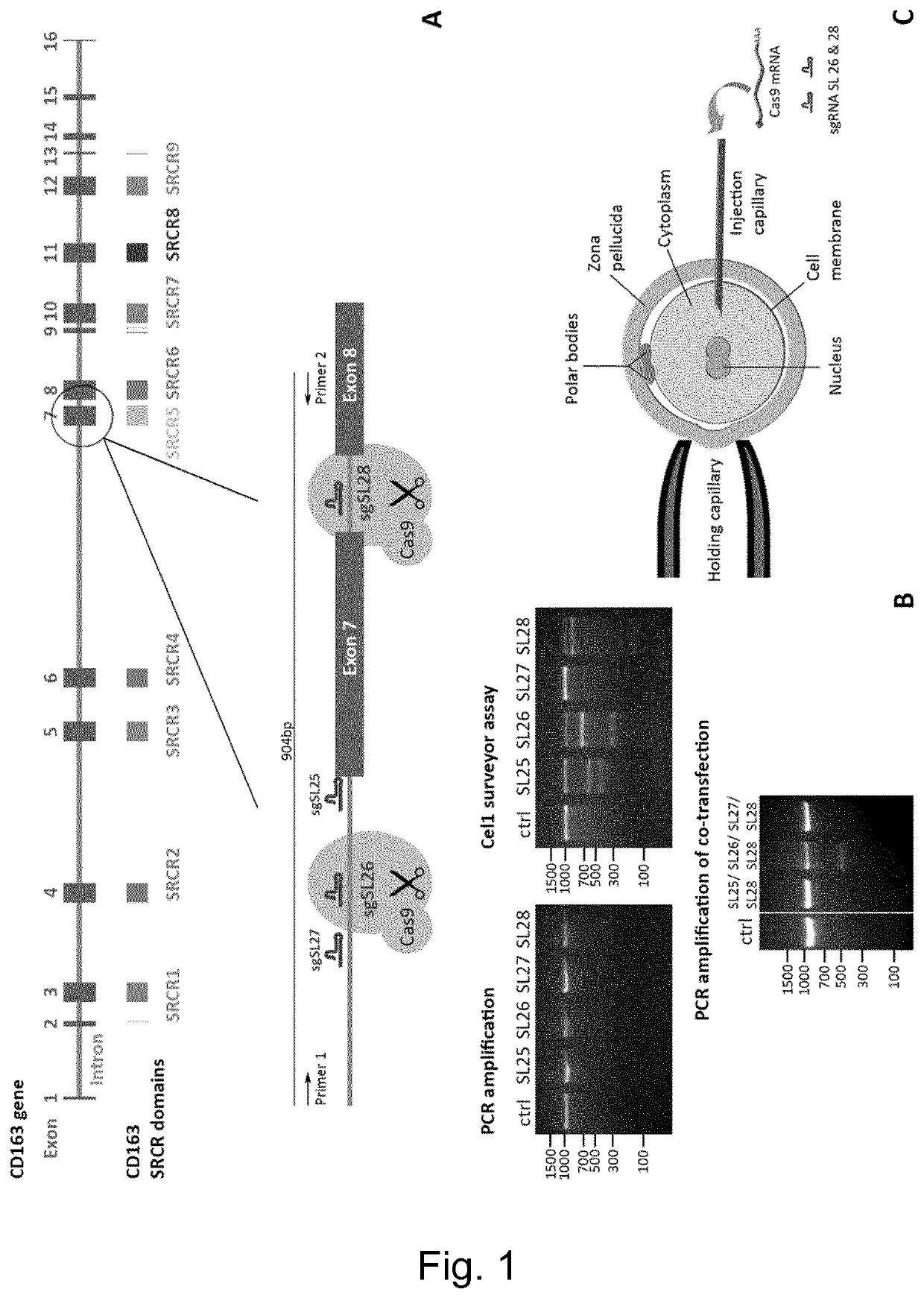
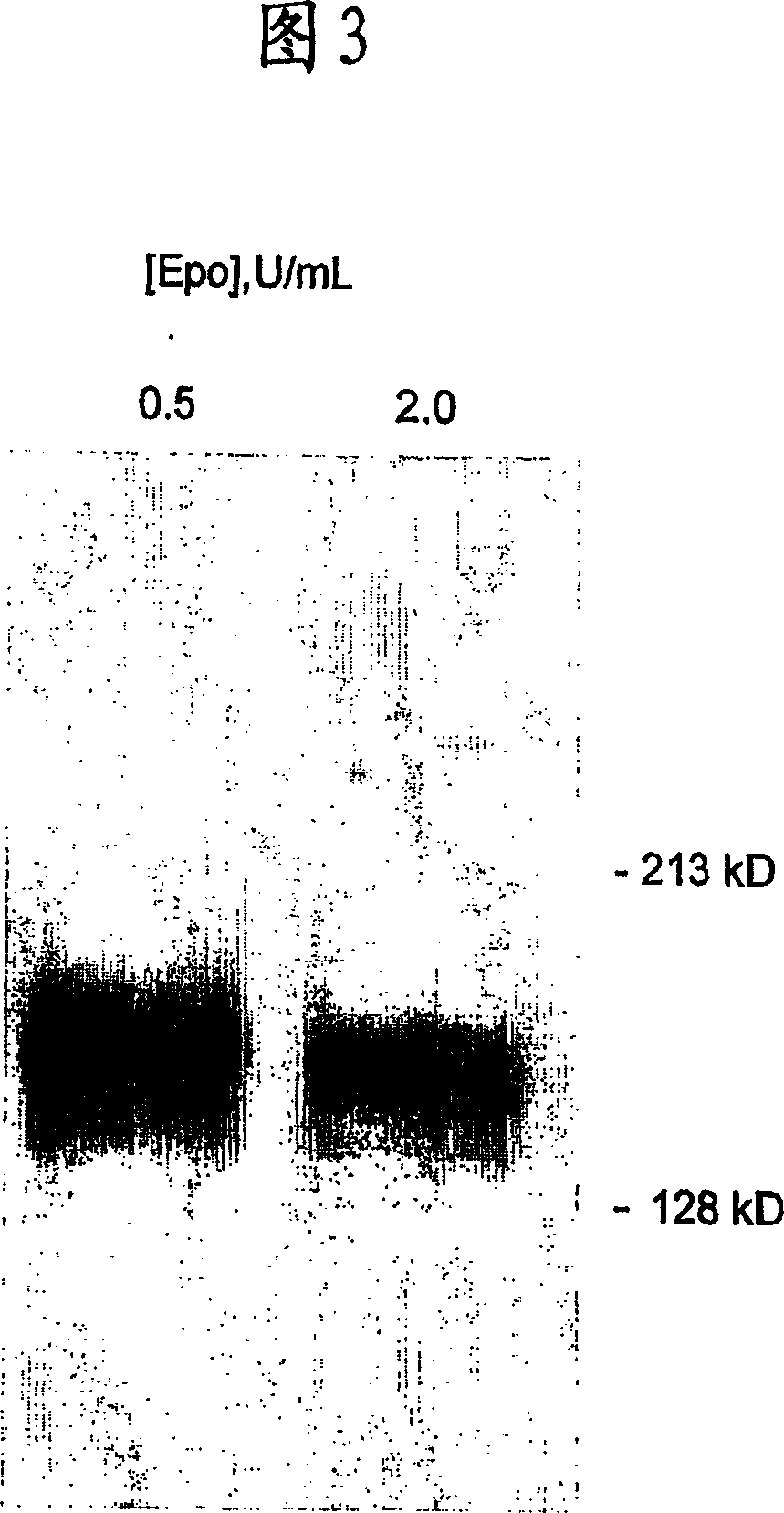
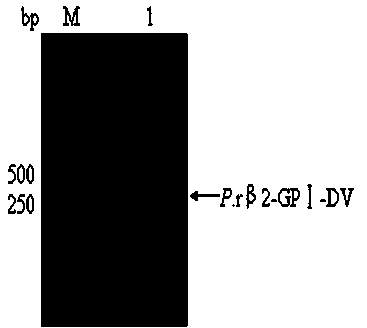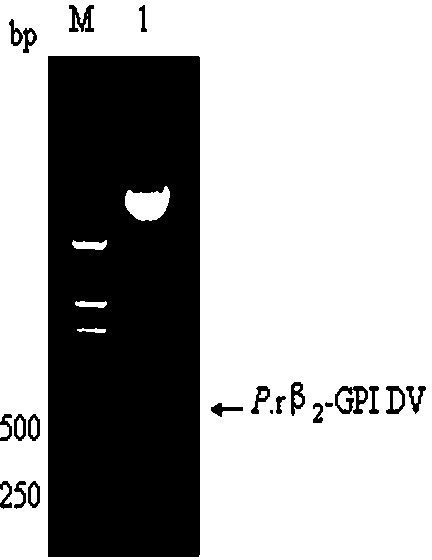Patents
Literature
65 results about "Scavenger receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Scavenger receptors are a group of receptors that recognize modified low-density lipoprotein by oxidation or acetylation. This naming is based on a function of cleaning: Scavenger receptors widely recognize and uptake macromolecules having a negative charge as well as modified LDL.
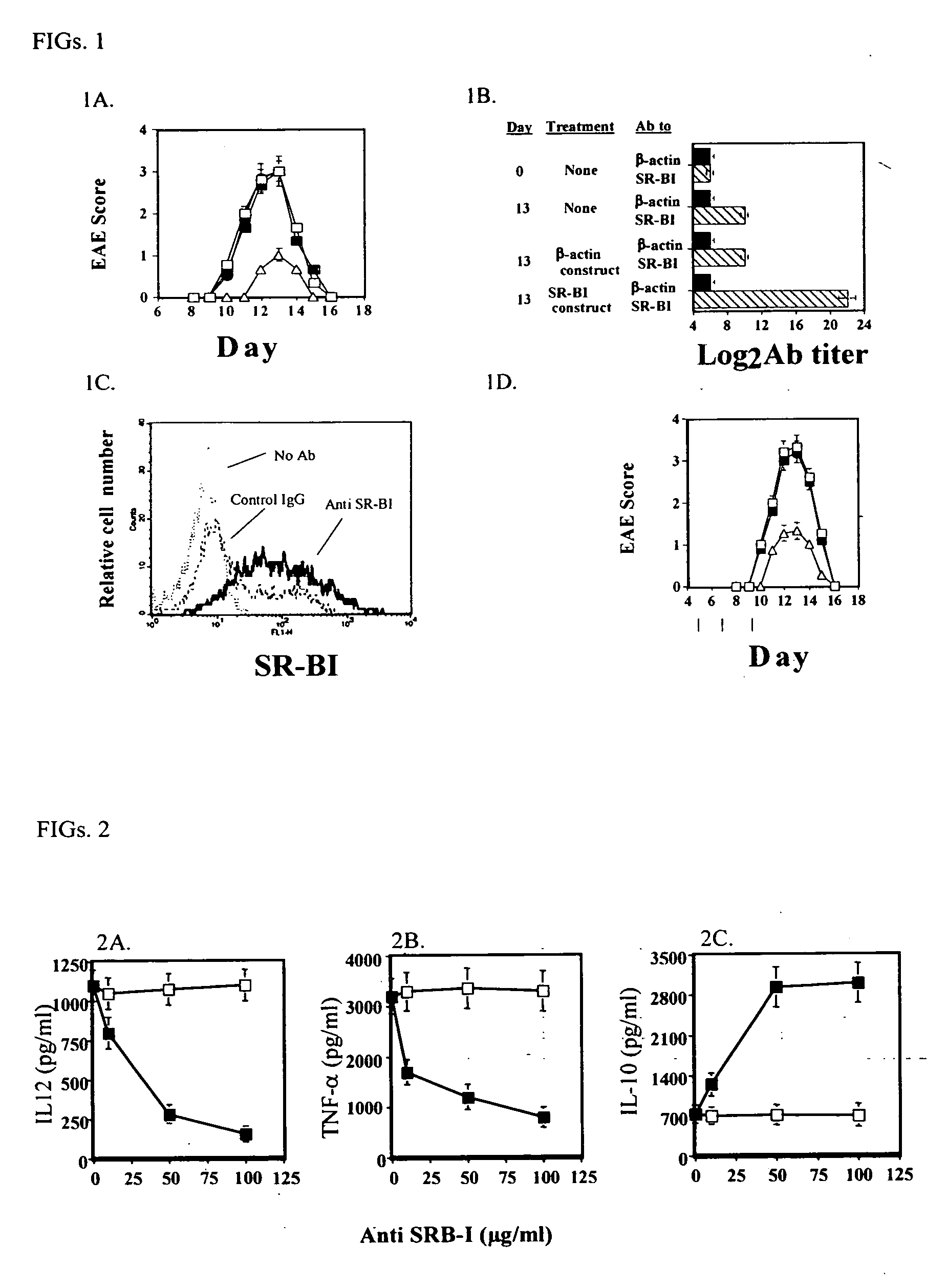
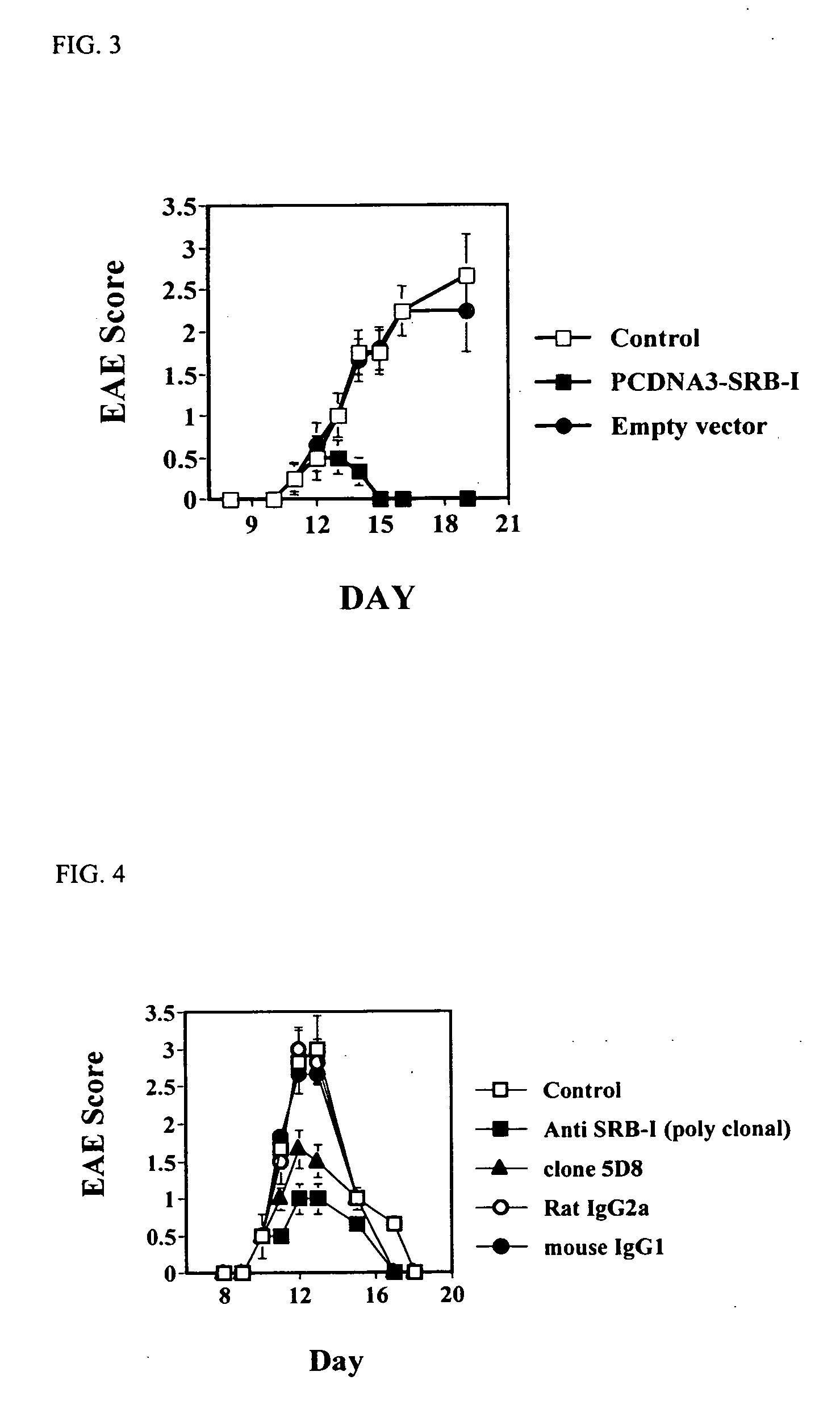
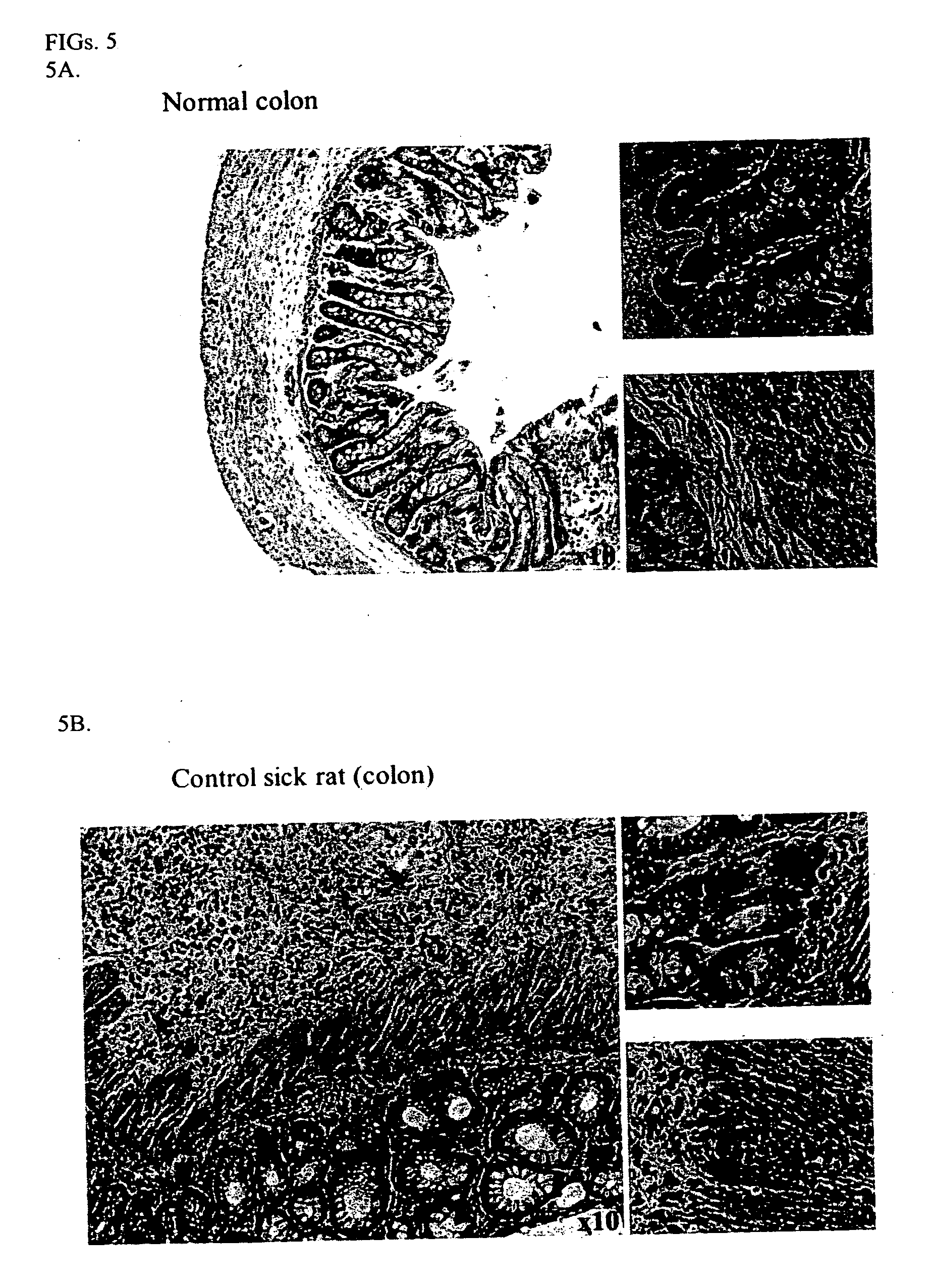
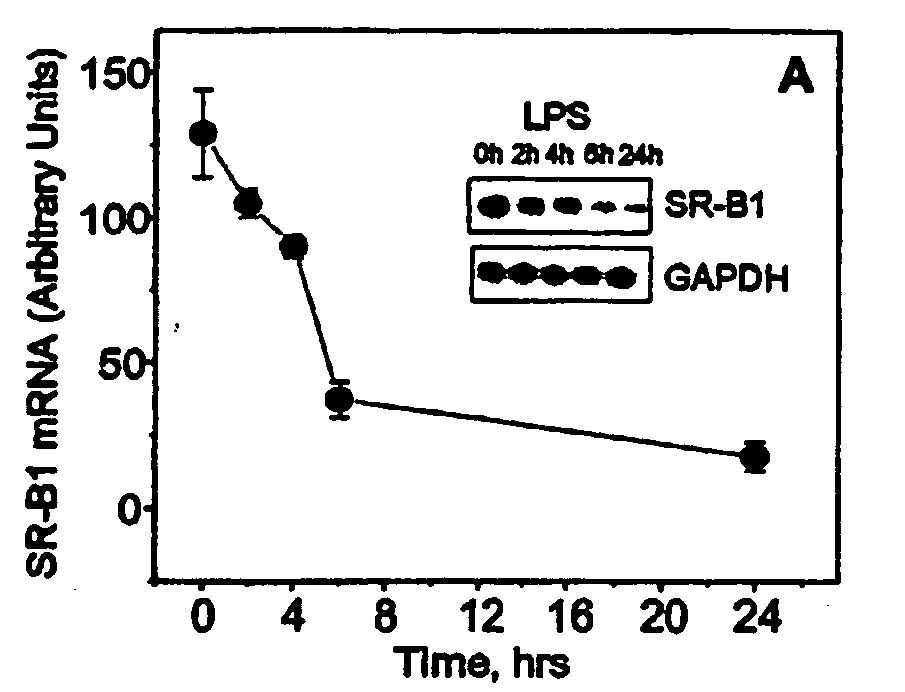
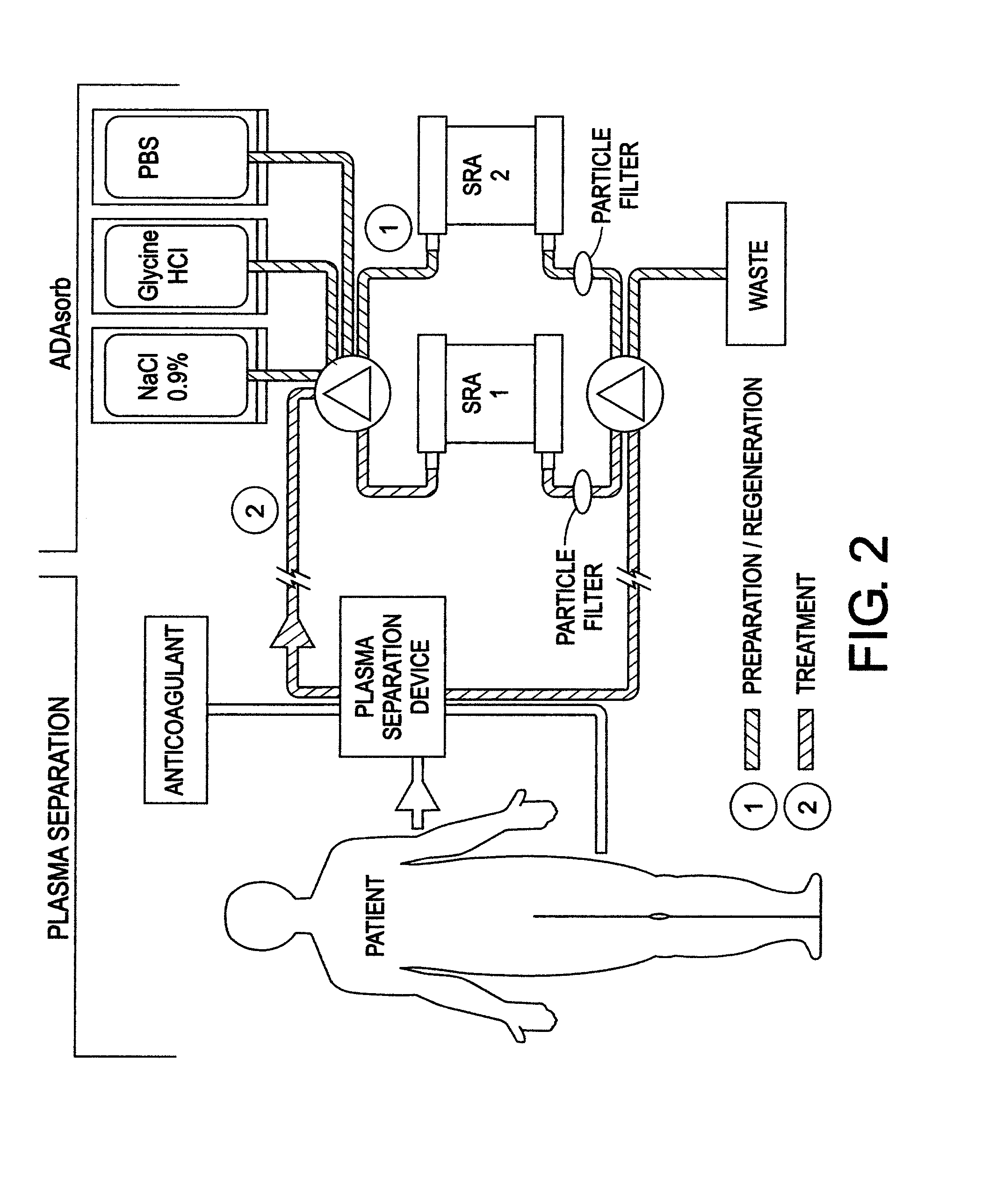
Compositions and methods for diagnosing and treating an inflammation
ActiveUS20060035834A1Reduce inflammationNervous disorderPeptide/protein ingredientsScavenger receptorInflammatory response
A method of reducing an inflammatory response in a subject is provided. The method comprising providing to a subject in need thereof a therapeutically effective amount of an agent capable of reducing activity and / or expression of a scavenger receptor or of an effector thereof, thereby reducing the inflammatory response in the subject.
Owner:TECHNION RES & DEV FOUND LTD
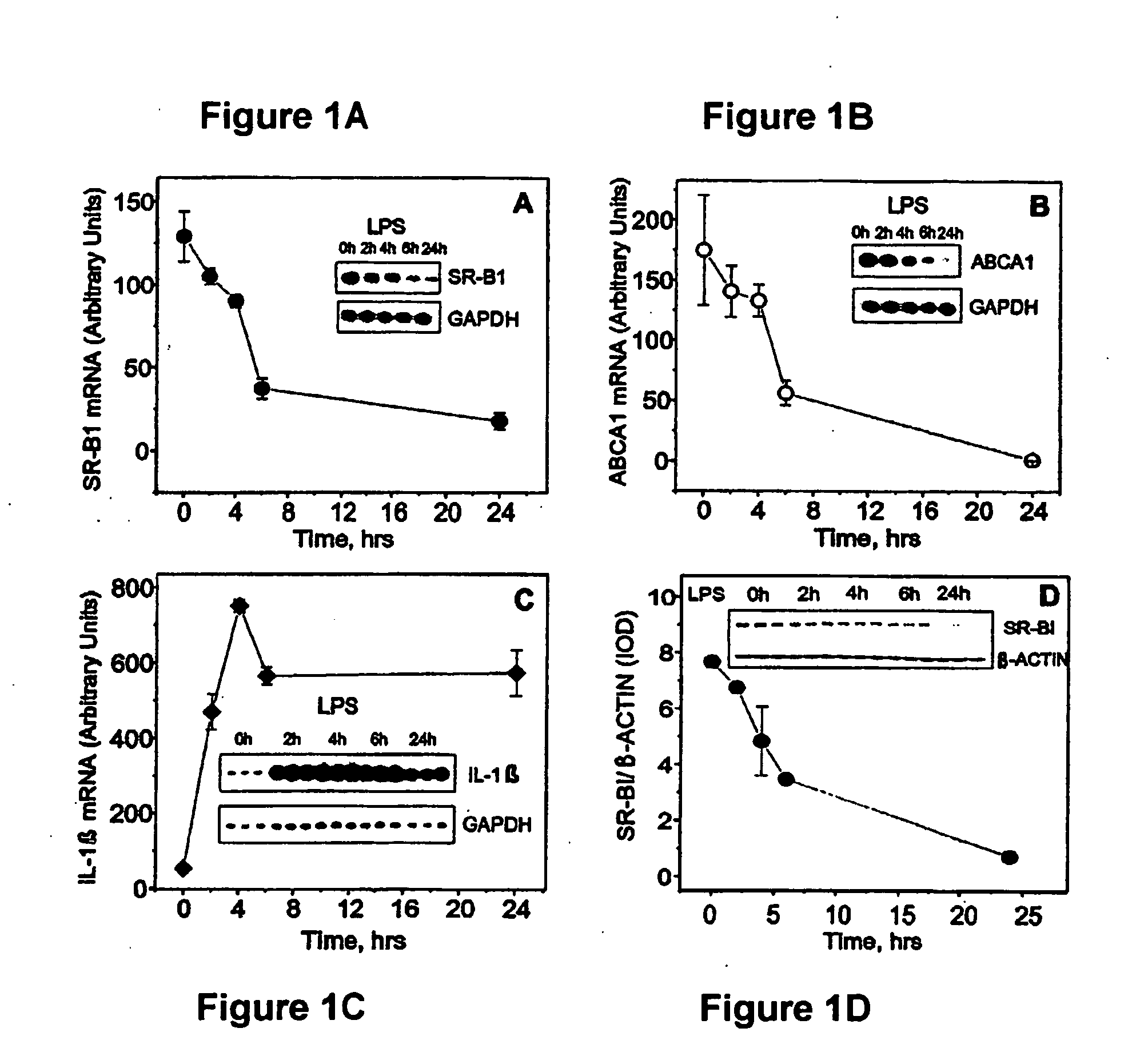
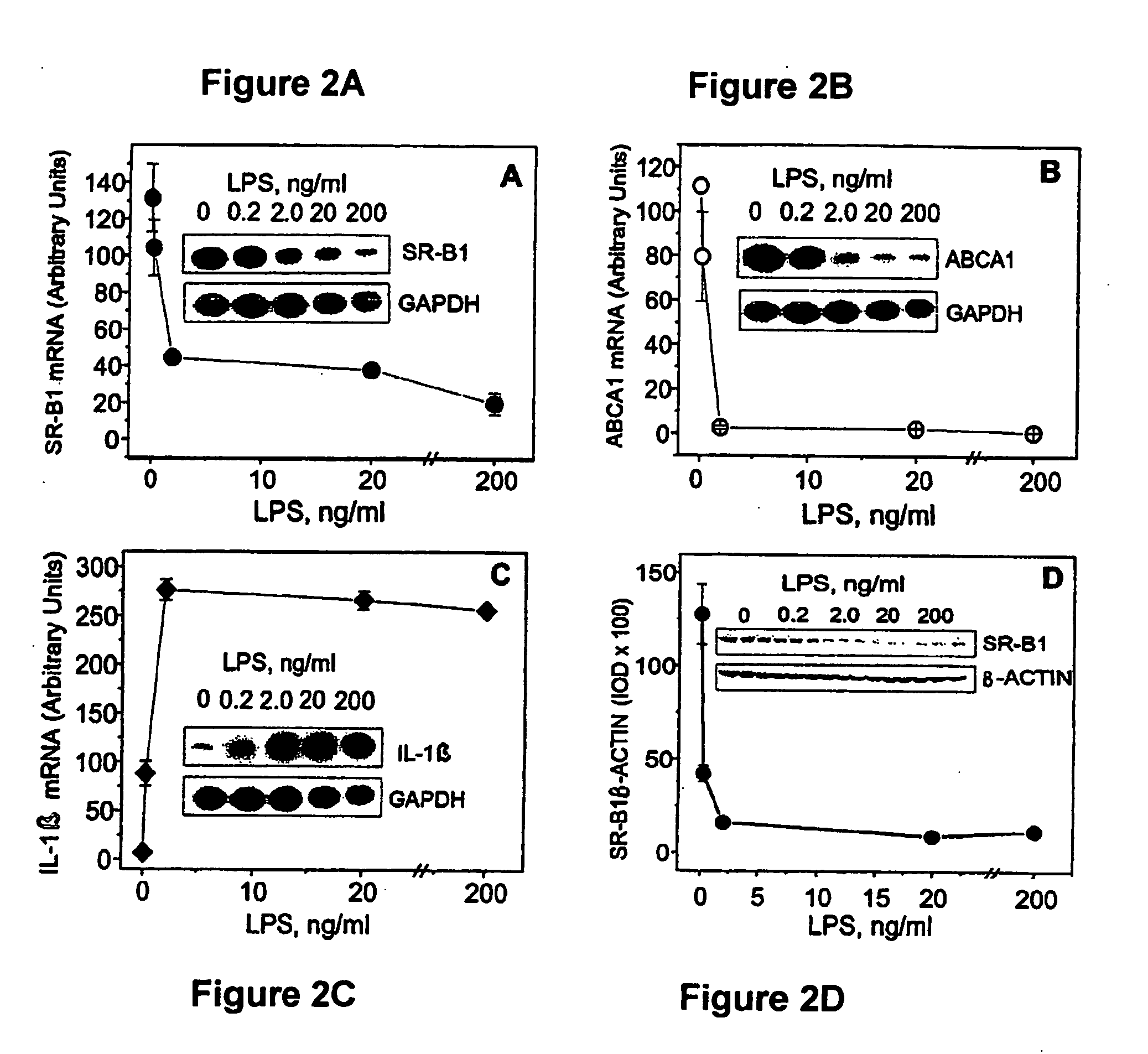
Scavenger Receptor B1 (Cla-1) Targeting for the Treatment of Infection, Sepsis and Inflammation
This invention relates to methods and compositions for the treatment of sepsis, inflammation or infection. In particular, the invention concerns the use of molecule(s) that target SR-BI, which is also referred to as CLA-1 (SR-BI / CLA-1), to treat sepsis, bacterial and viral infections, and inflammatory diseases. SRB I / CLA-1 ligands contributing to the pathogenesis of disease include LPS, LTA, viral envelope proteins, beta-amyloid, serum Amyloid A and / or heat shock proteins.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NIH
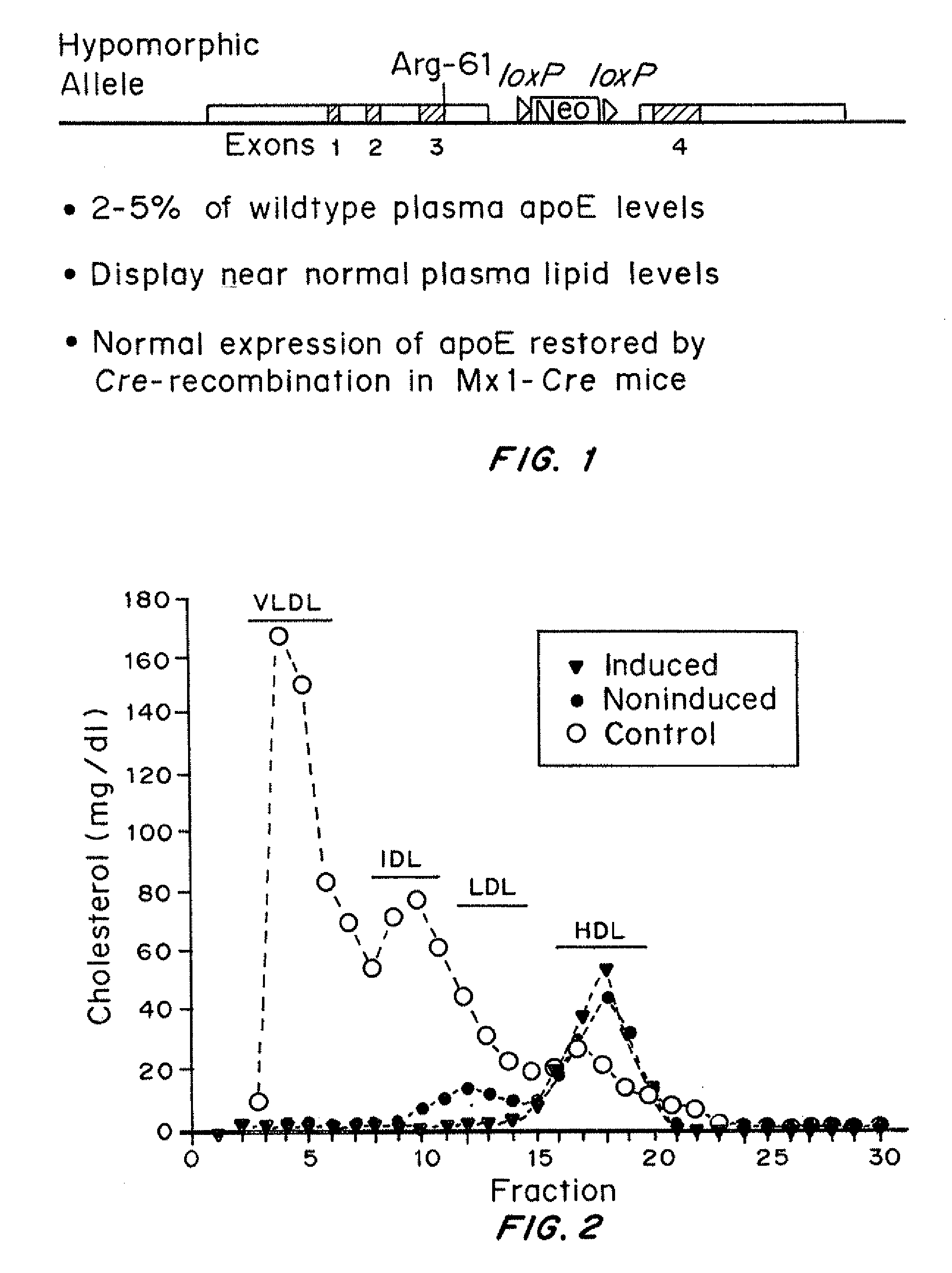
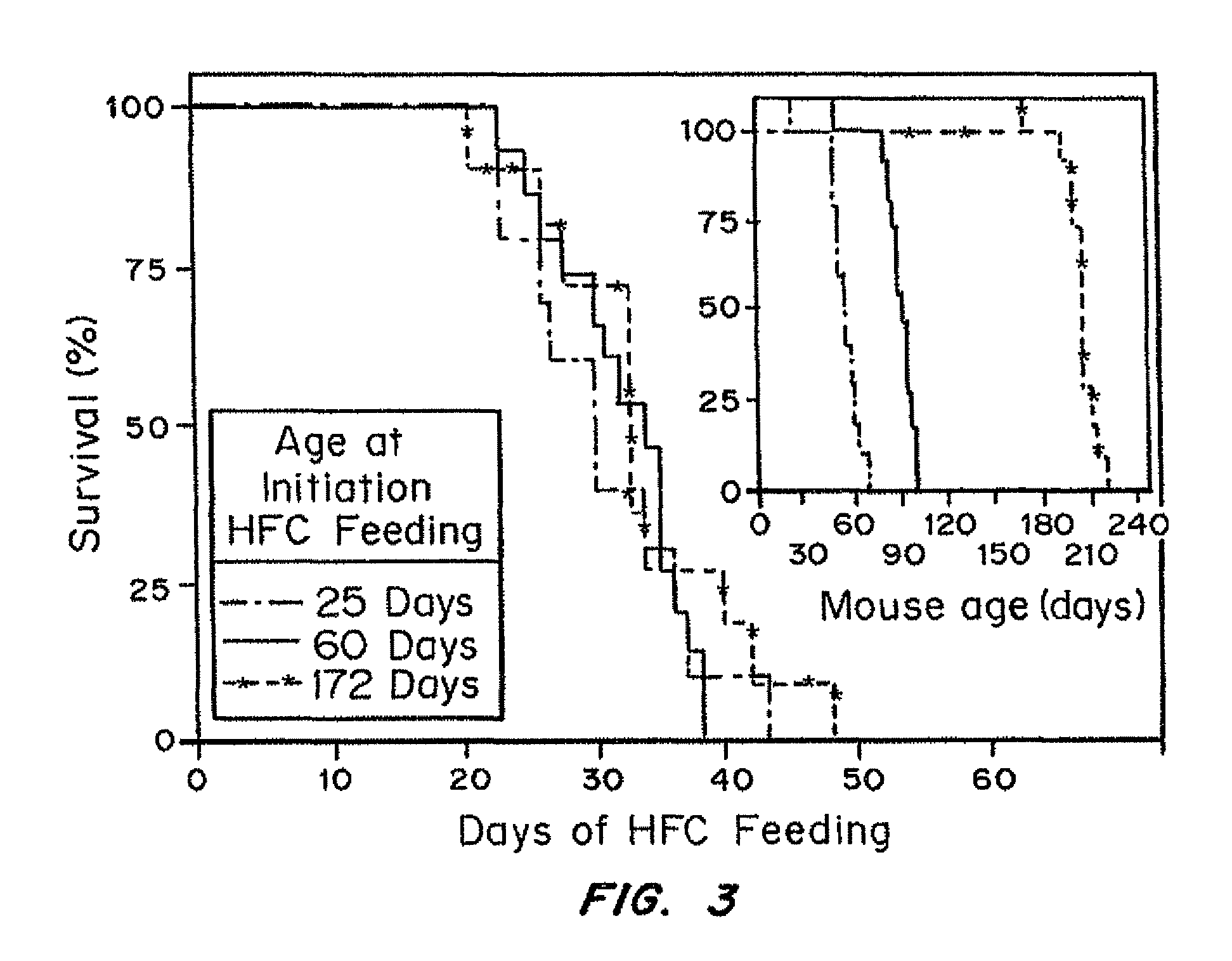
Inducible heart attack animal model
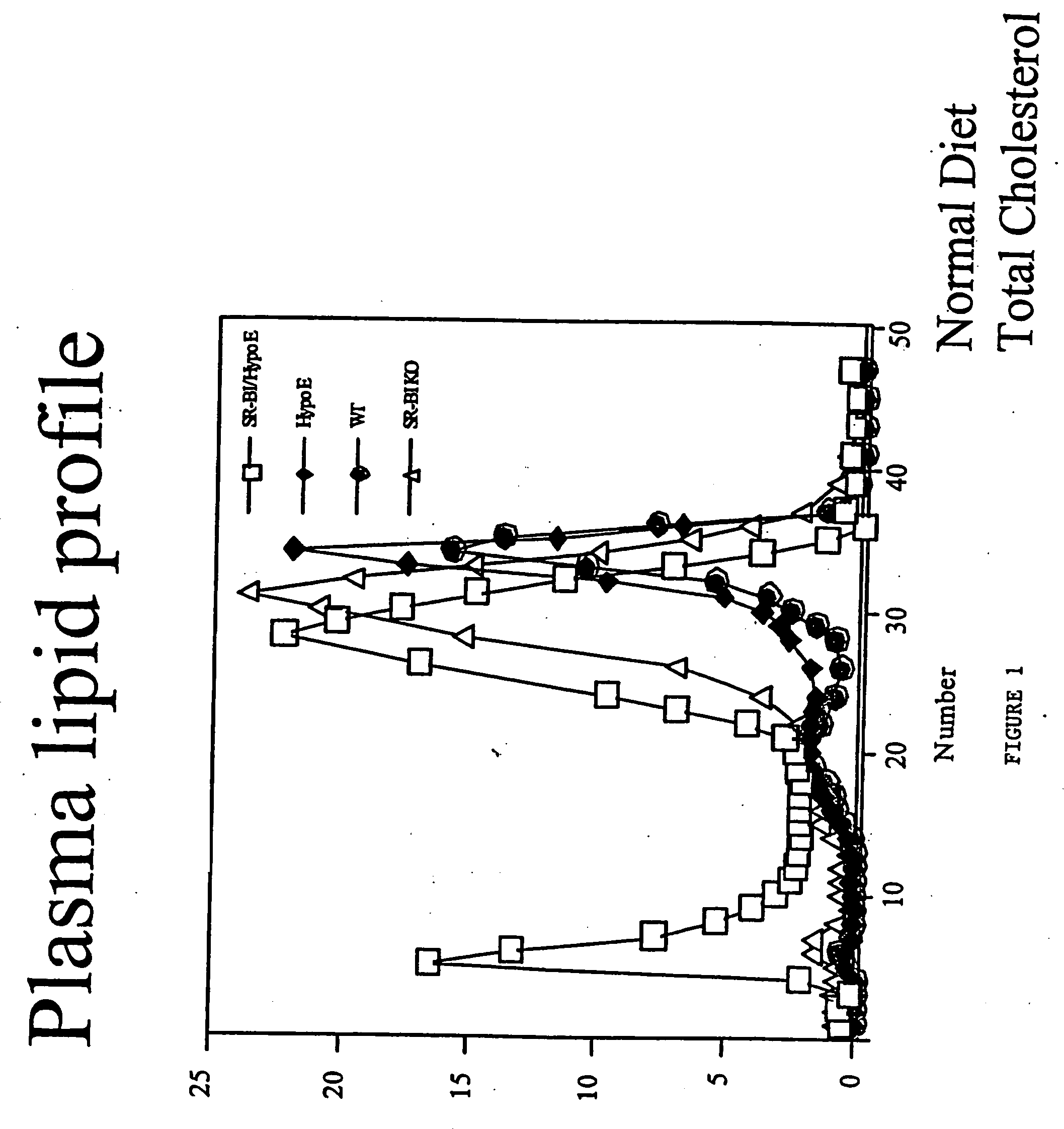
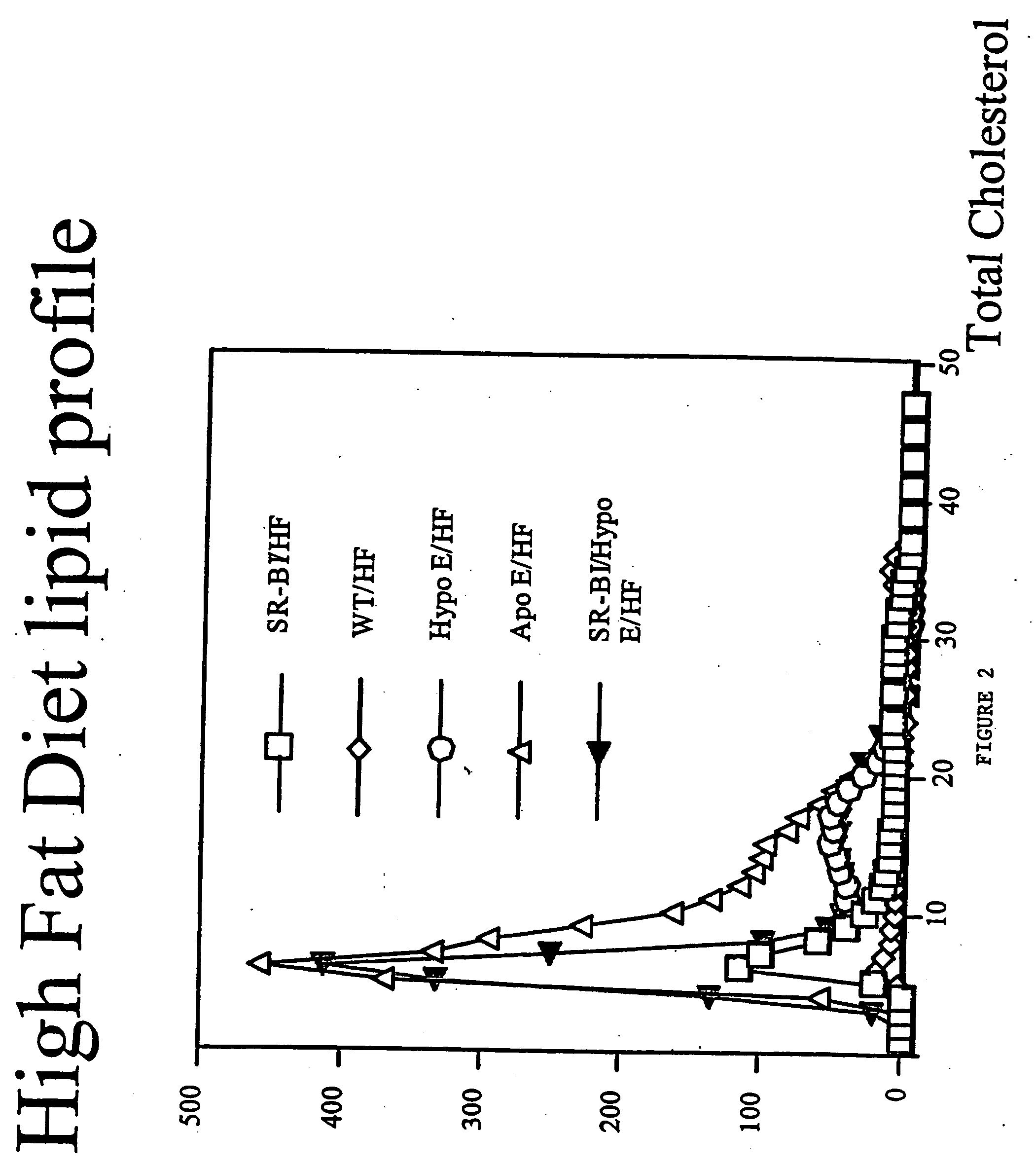
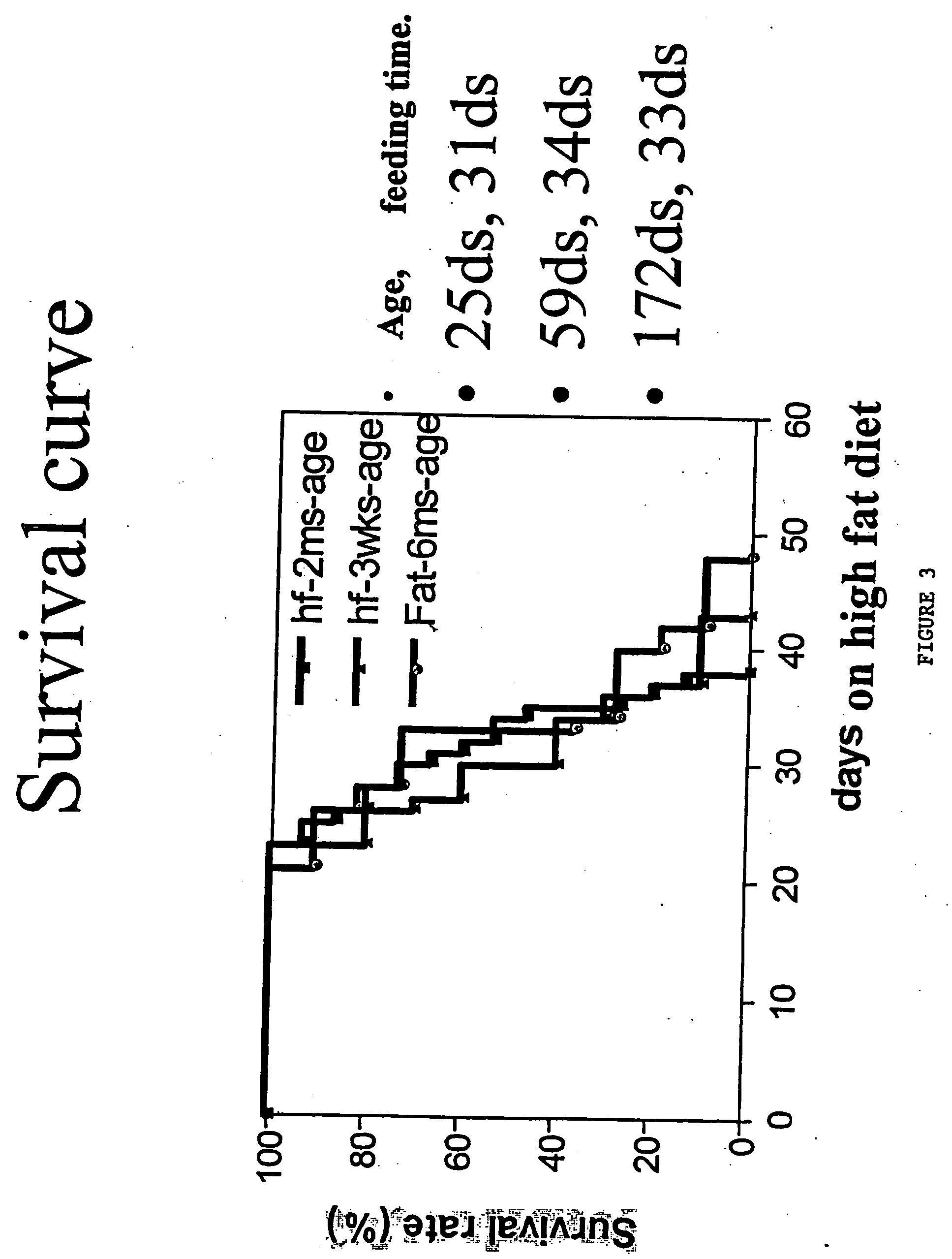
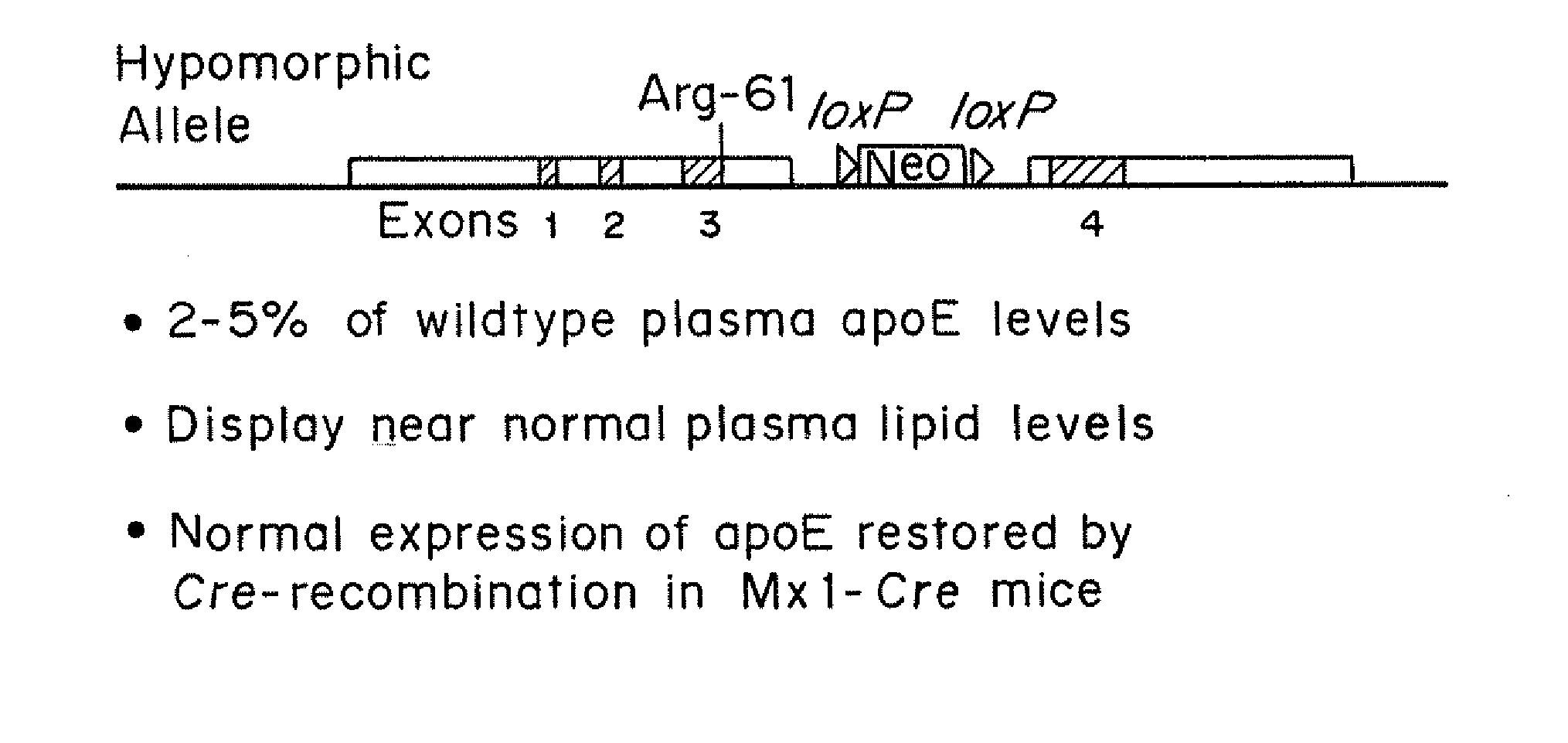
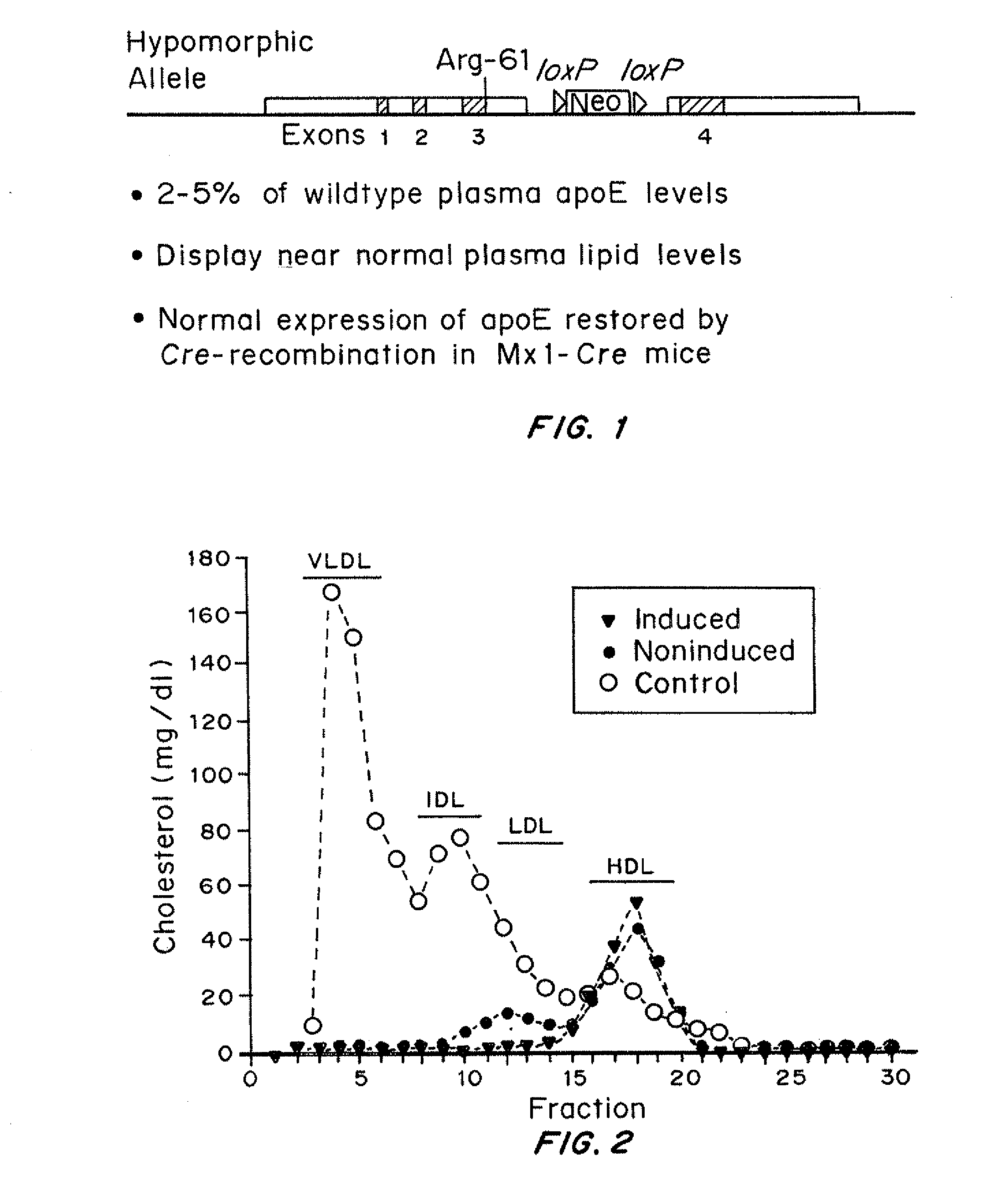
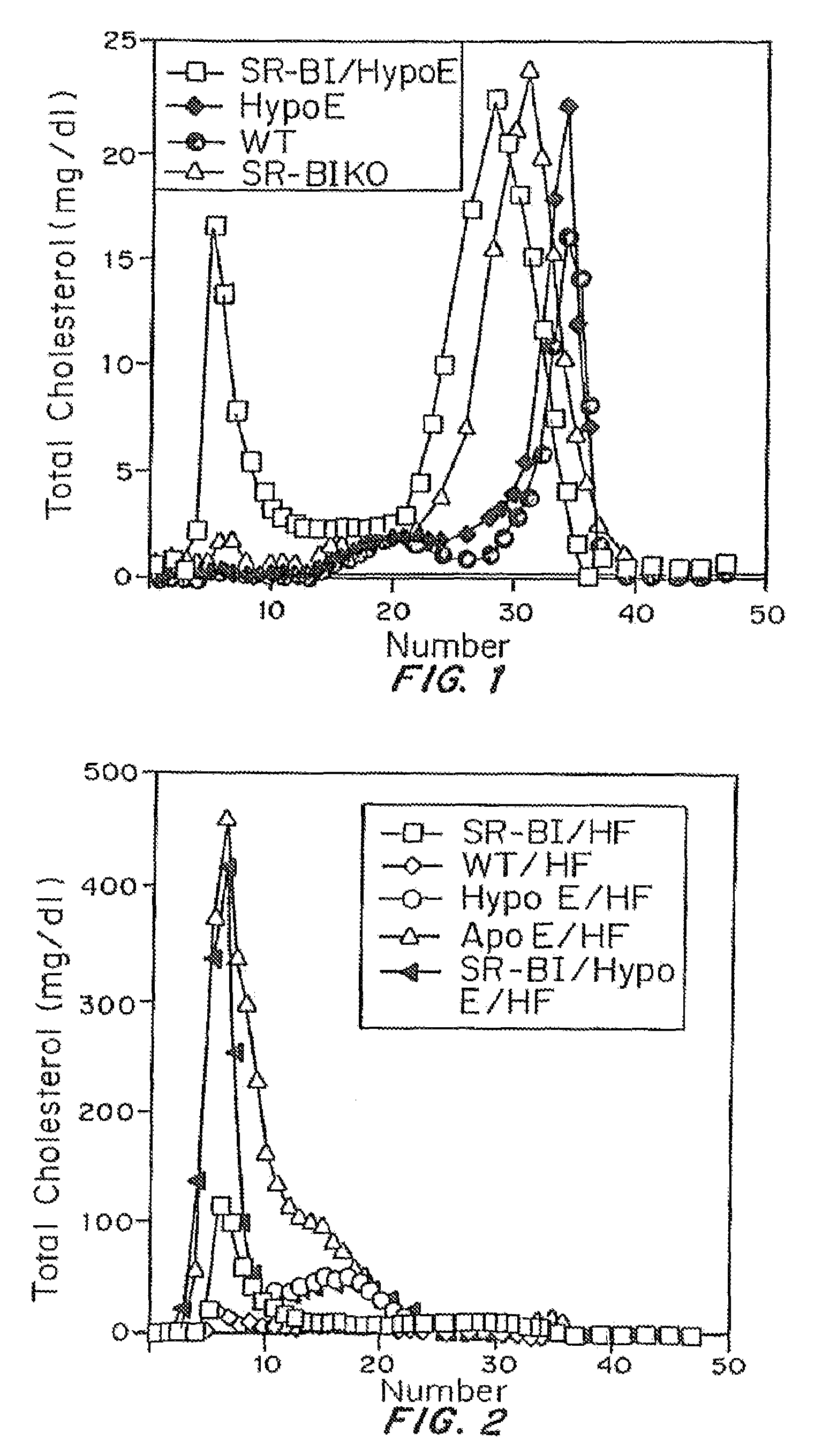
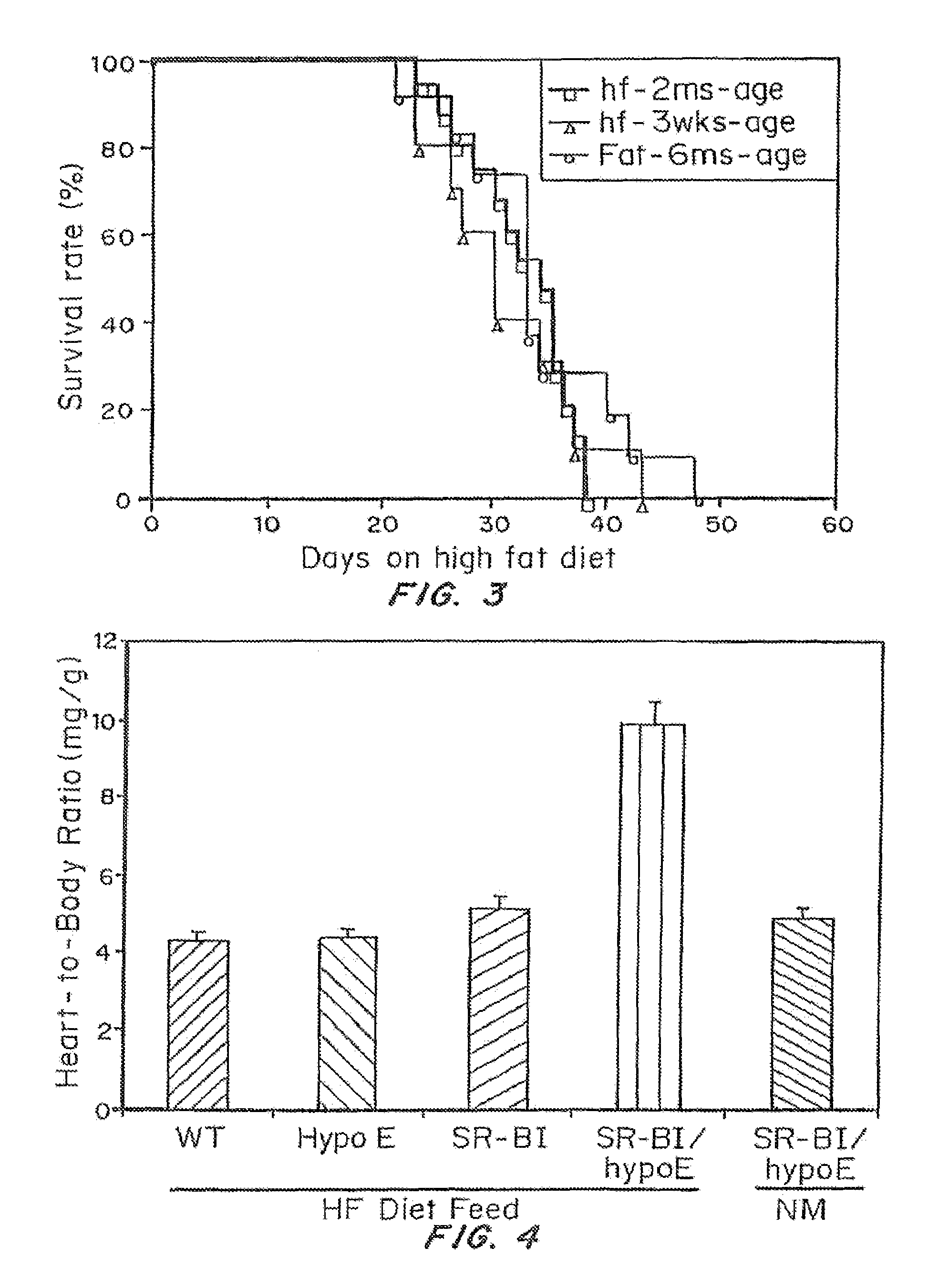
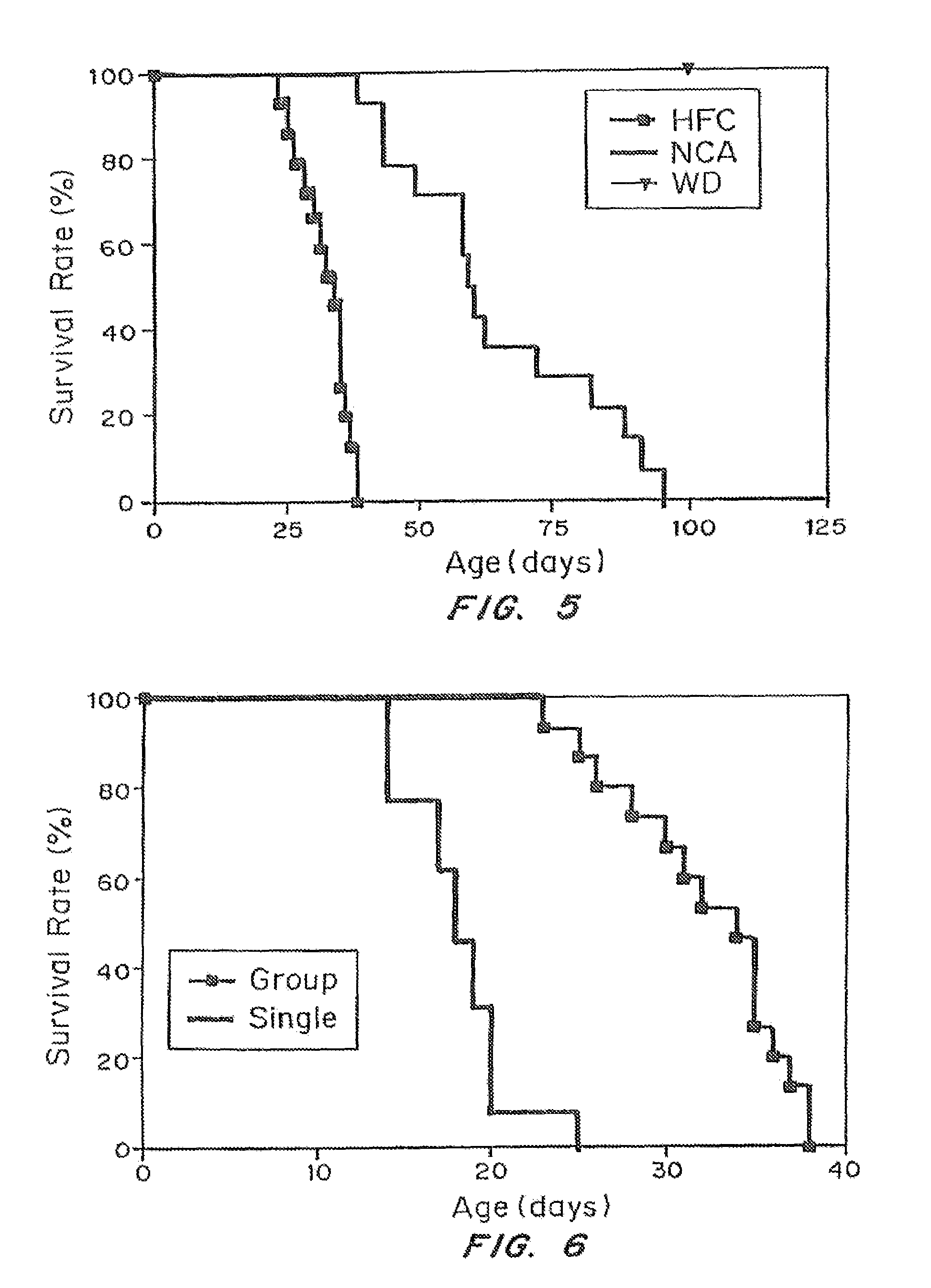
An animal model of coronary heart disease has been developed where myocardial infarct can be induced by altering the animal's diet. In all embodiments, this animal model is a result of reduced activity of scavenger receptor class BI (SR-BI) and apolipoprotein E (ApoE). In a preferred embodiment, the model is a result of crossbreeding two transgenic mouse lines: a knockout of SR-BI (SR-BI− / −) and an impaired ApoE expressor (hypoE). The impaired ApoE gene results in only 2-5% expression of ApoE and a reduction in cholesterol homeostasis. Resulting animals are predisposed to hypercholesterolemia but can live longer than a year on a normal low fat diet. Serum plasma levels can be significantly elevated by changing the animal's diet to one containing high levels of fat and cholesterol. Within a month on a high fat, high cholesterol diet, animals develop atherosclerosis and myocardial infarction occurs. Survival depends on the nature of the diet and the conditions of animal husbandry and can typically be around 20-30 days after administration of the modified diet depending on the specific conditions. Housing the animals alone or in groups significantly affects survival of these animals on a high fat diet. Analysis of B- and T-cell deficient SR-BI / ApoE / RAG2 triple knockout mice established that B- and T-lymphocytes do not play a key role in the pathophysiology of the SR-BI ApoE dKO model of human disease. These animal models can be used to study mechanisms and progression of CHD as a function of diet, treatment with drugs to be screened for efficacy or undesirable side effects, and social environmental effects.
Owner:MASSACHUSETTS INST OF TECH
Preparation and application of gene and chemotherapeutic drug co-delivery anti-tumor nanoparticle of target SR-BI (Scavenger Receptor-BI)
InactiveCN103816122AHigh encapsulation efficiencyTargetedPowder deliveryOrganic active ingredientsTumor targetCytotoxicity
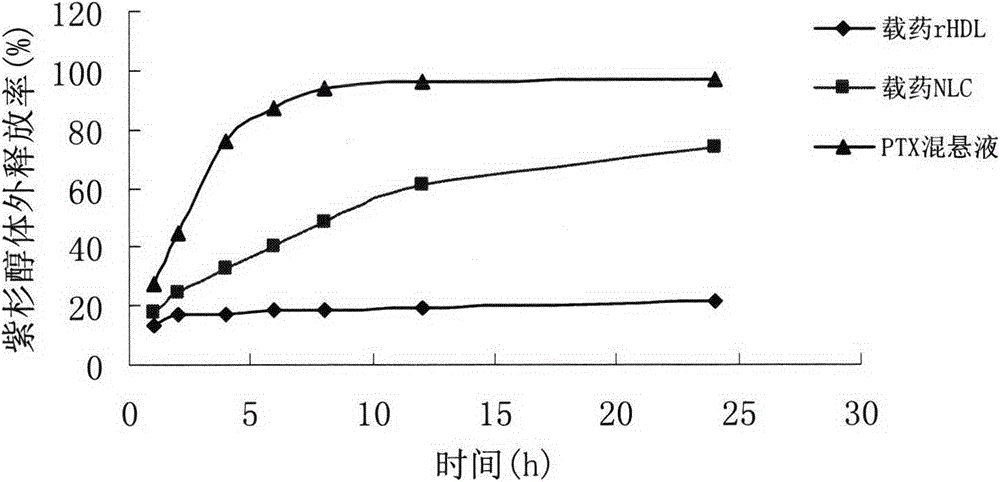
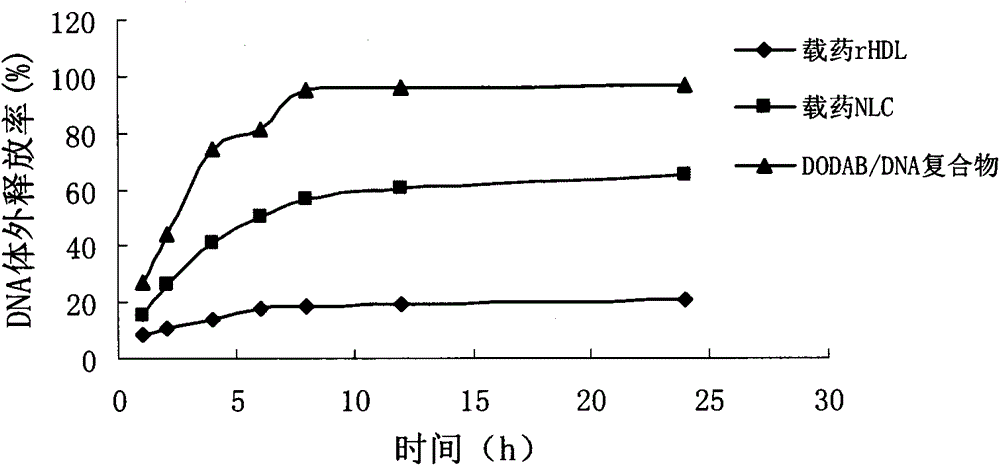
The invention relates to preparation and application of a gene and chemotherapeutic drug co-delivery anti-tumor nanoparticle of a target SR-BI (Scavenger Receptor-BI). The nanoparticle is a recombinant high density lipoprotein drug-loaded nanoparticle, comprises phospholipid, cholesterol, cholesteryl ester, apolipoprotein, cation lipid, a chemotherapeutic drug, a gene drug and the like, and is prepared by a film dispersion method. The invention aims at preparing the bionic tumor target drug-loaded nanoparticle, the preparation condition is mild, and the cost is low. The invention further provides the application of the gene and chemotherapeutic drug co-delivery anti-tumor nanoparticle. The gene and chemotherapeutic drug co-delivery anti-tumor nanoparticle has the advantages of spherical structure, good encapsulation efficiency, low cytotoxicity, good targeting, high safety, significant in-vitro anti-tumor effect and the like.
Owner:CHINA PHARM UNIV
Methods to identify therapeutic agents
InactiveUS20070059242A1Increase lipid-loadingLittle effectBiocideMetabolism disorderE-selectinDisease
As illustrated herein, cholesterol is oxidized when it is present in atherosclerotic plaques. This reaction generates cholesterol oxidation or ozonation products that can act as chemotactic attractants of macrophages, can promote differentiation of monocytes into macrophages and can increase expression of E-selectin and Class A scavenger receptor (SR-A). The present application is directed to methods of using such cholesterol ozonoation products to identify agents that can be used to treat atherosclerosis and other inflammatory artery diseases.
Owner:THE SCRIPPS RES INST
Growth-related molecular marker in C-type scavenger receptor of Litopenaeusvannamei and application
ActiveCN109055580AImprove selection efficiencyImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationCandidate Gene Association StudyReceptor for activated C kinase 1
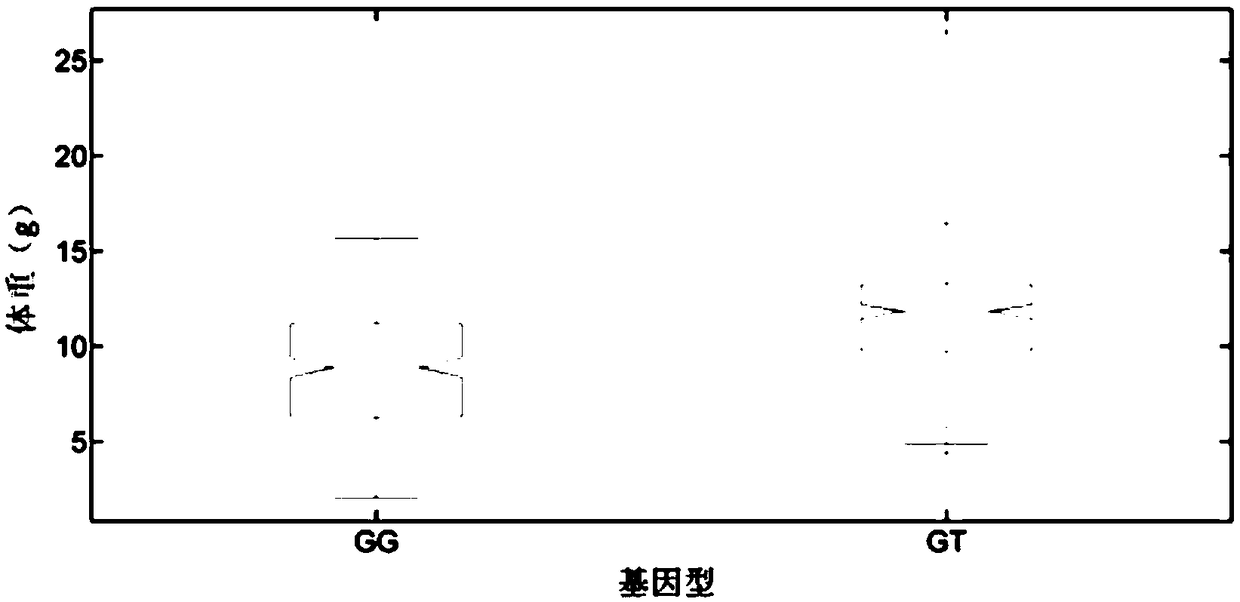
The invention belongs to the technical field of aquatic breeding, and particularly relates to a growth-related molecular marker in a C-type scavenger receptor gene (LvSRC) of Litopenaeusvannamei and application thereof to genetic breeding of the Litopenaeusvannamei. A batch of markers related to shrimp growth traits are positioned in the genomic level through genome-wide association analysis, a single nucleotide polymorphism (SNP) marker (LvSRC-211-G / T) positioned in a coding region of the LvSRC gene is further screened through gene annotation and by a candidate gene association analysis method, and the genotype of the marker is significantly correlated to the shrimp growth traits (P is smaller than 10<-6>). The marker is positioned at a 211bp position on the LvSRC gene and belongs to G / Ttype mutation, and the GT genotype of the marker is a distinct growth-dominant genotype. The marker provided by the invention can be used as the shrimp breeding molecular marker for assisting in genetic selection of the shrimp growth traits.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
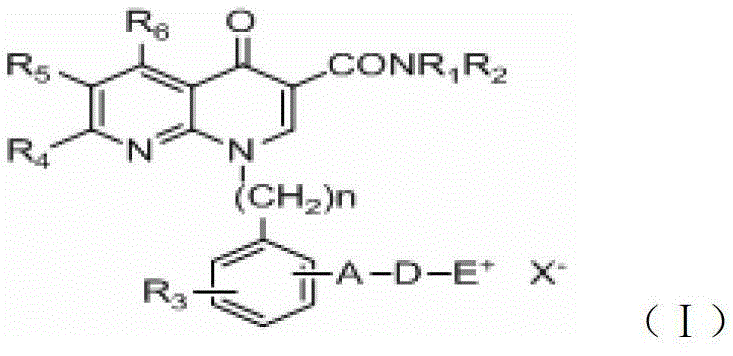
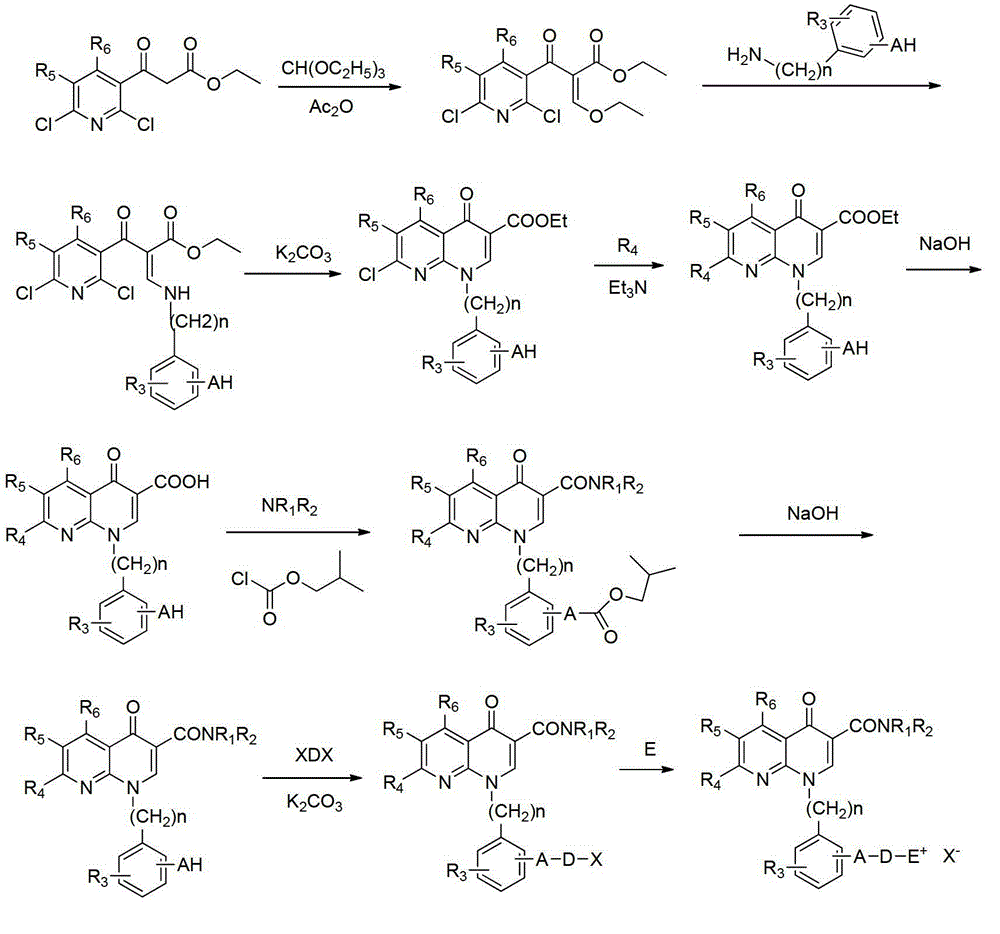
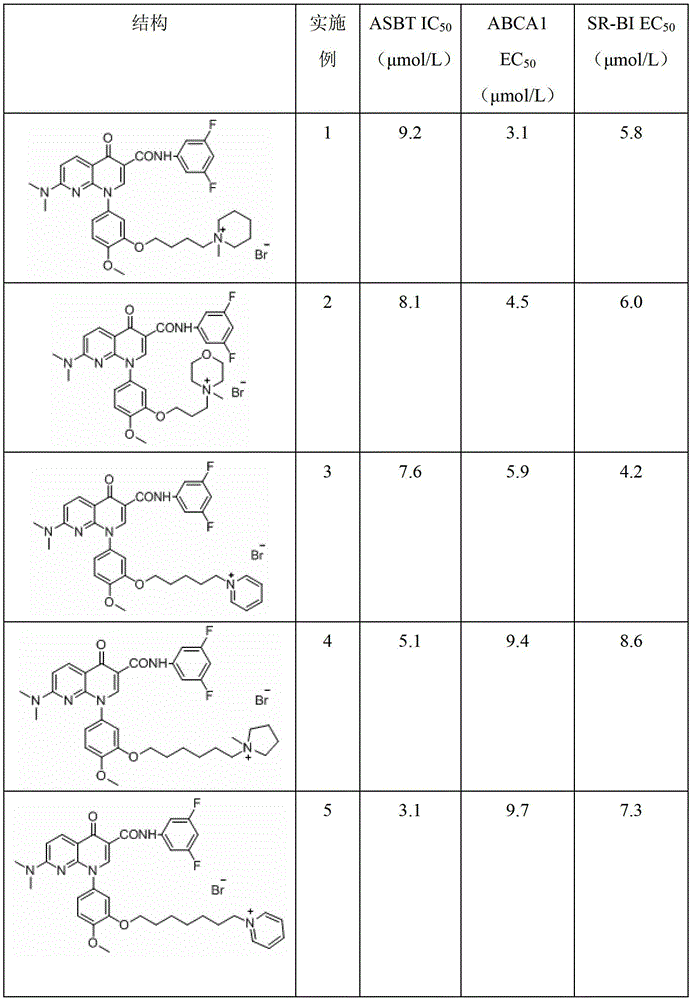
A group of 1-substituted-1, 8-naphthyridine formamide derivatives and preparation and application thereof
InactiveCN103183676AOrganic active ingredientsOrganic chemistryScavenger receptorATP-Binding Cassette Transporter A1
The invention relates to a group of 1-substituted-1, 8-naphthyridine formamide derivatives, and a preparation method and an application thereof. The biological experimental studies show that the tested compounds in the derivatives remarkably inhibit the apical sodium-dependent bile acid transporter (ASBT) at the cellular level, and have increased effect on the expression of ABCA1 (ATP-binding cassette transporter A1) and SR-BI (scavenger receptor type B class I), thereby being expected to be developed to become a clinically effective medicament for treating associated cardiovascular diseases.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Mouse Model of Chronic Heart Failure and Coronary Atherosclerosis Regression
ActiveUS20080075663A1Reduced activityRestore blood flowCompounds screening/testingApolipeptidesScavenger receptorLower blood cholesterol
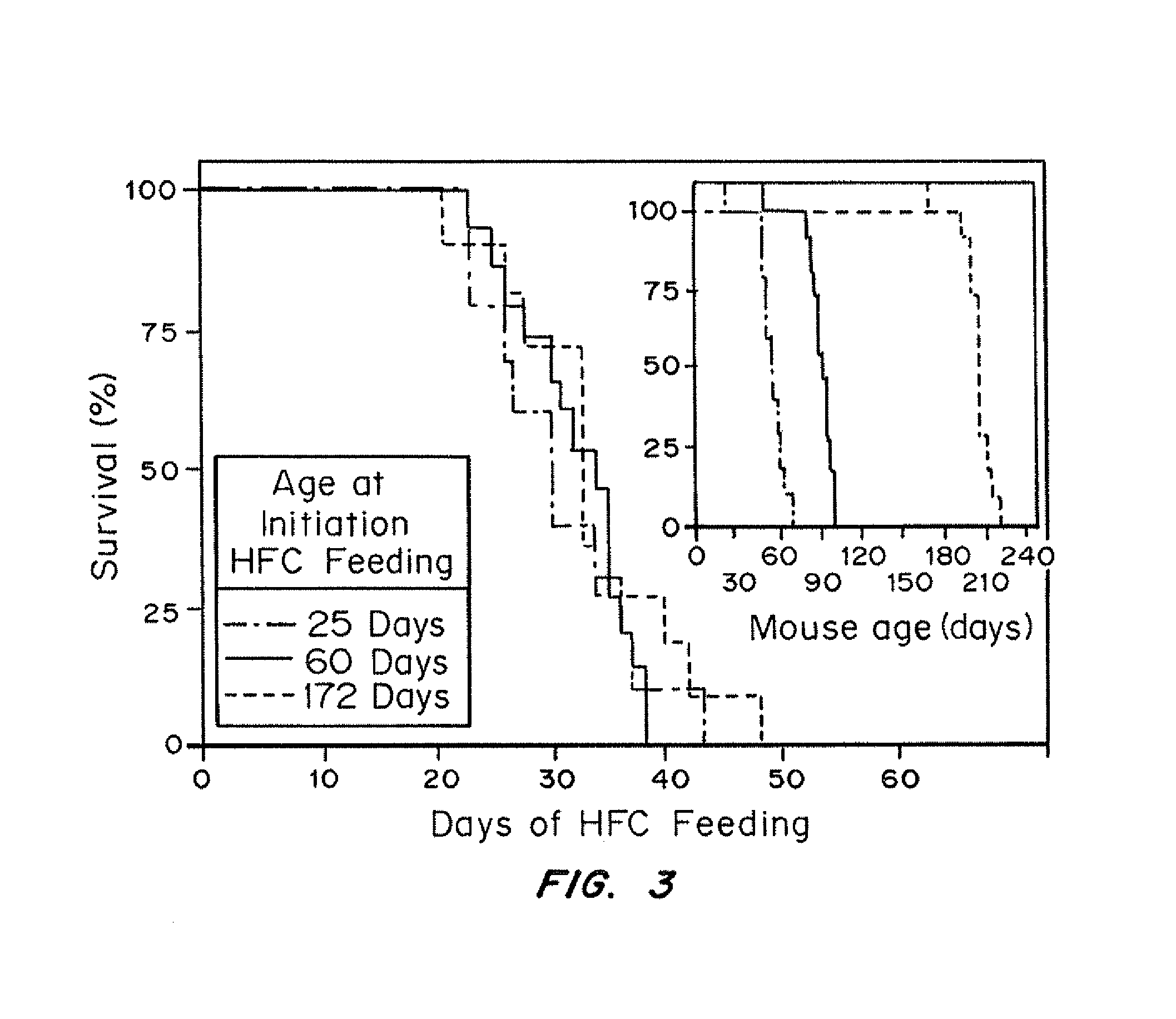
An animal model has been developed where the animals can survive myocardial infarctions caused by diet-induced coronary atherosclerosis, and live with chronic heart failure. This animal model is a result of reduced activity of scavenger receptor class BI (SR-BI) and ApoE and the inducible activity of the Mx1-Cre gene. In a preferred embodiment, the model is a result of crossbreeding two transgenic mouse lines: a knockout of SR-BI (SRBI− / −) and an impaired ApoE expressor (Apoeh / h) to generate a strain referred to as Apoeh / hSRB1− / − mice, which is then crossbred to mice that carry the inducible Mx1-Cre transgene. The Apoeh / hSRB1− / − mouse model is genetically modified, enabling the offspring to rapidly and permanently lower their high blood cholesterol levels caused by dietary challenge. The ability to rapidly and permanently lower blood cholesterol levels in these mice stops and may cause the regression of occlusive coronary atherosclerosis restoring blood flow to the heart, allowing the mice to survive from myocardial infarction and live with chronic heart failure.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +2
Multi-mode ferritin nanometer contrast agent as well as preparation method and application thereof
ActiveCN108478810AAchieve synthesisEnable accurate diagnosisPowder deliveryLuminescence/biological staining preparationScavengerFluorescence
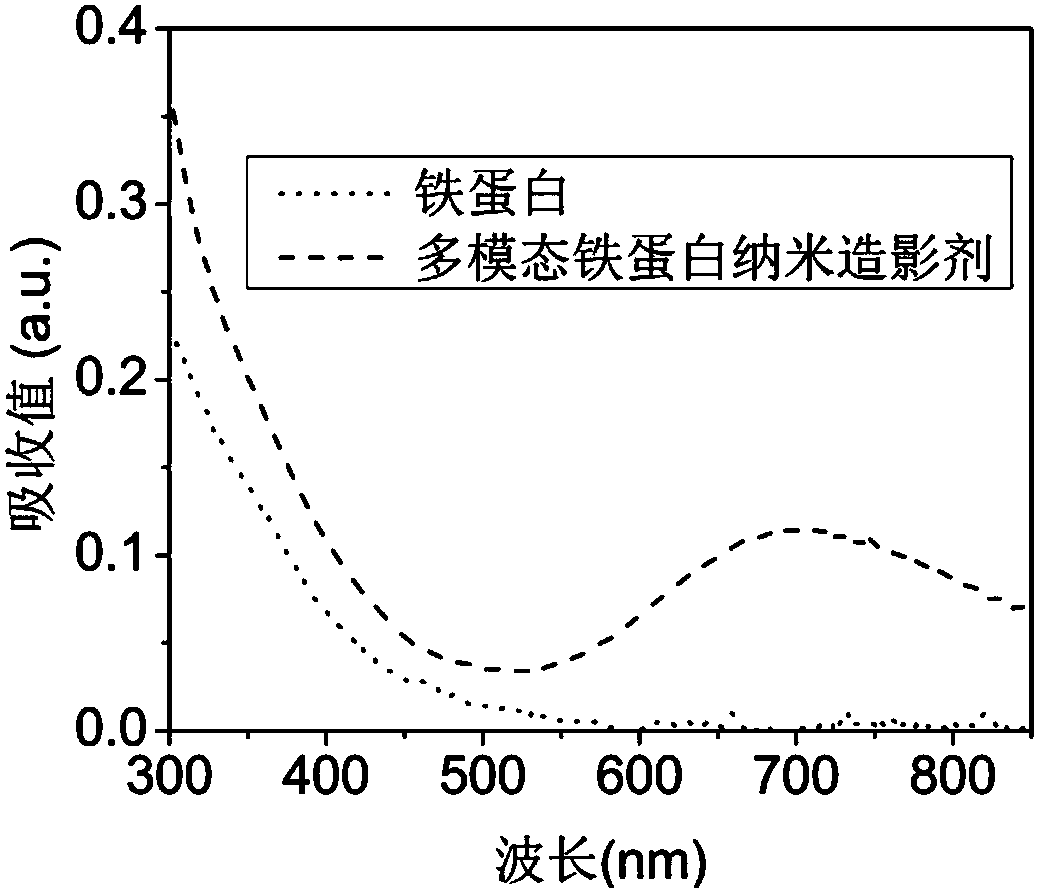
The invention discloses a multi-mode ferritin nanometer contrast agent based on ferritin and a preparation method thereof. Horse spleen ferritin with the targeted performance on macrophage surface scavenger receptors 5 is used as a biological nanometer template (shell); prussian blue nanometer particles are used as cores to realize MRI and photoacoustic imaging; next, fluorescent dye IR-782 is coupled to be used as a fluorescence probe part; the multi-mode ferritin nanometer contrast agent of a shell core structure is prepared. The multi-mode ferritin nanometer contrast agent combines the photoacoustic imaging, fluorescence imaging and MRI trimodal imaging, can be used for sentinel lymph node photoacoustic / MRI / fluorescence trimodal radiography before breast cancer, can also be used for performing blue dyeing on the sentinel lymph node before breast cancer by using the dyeing effect of the prussian blue, realizes the comprehensive and precise diagnosis of the breast cancer lymphatic metastasis, and promotes the development of clinic medicine and image diagnostics.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV
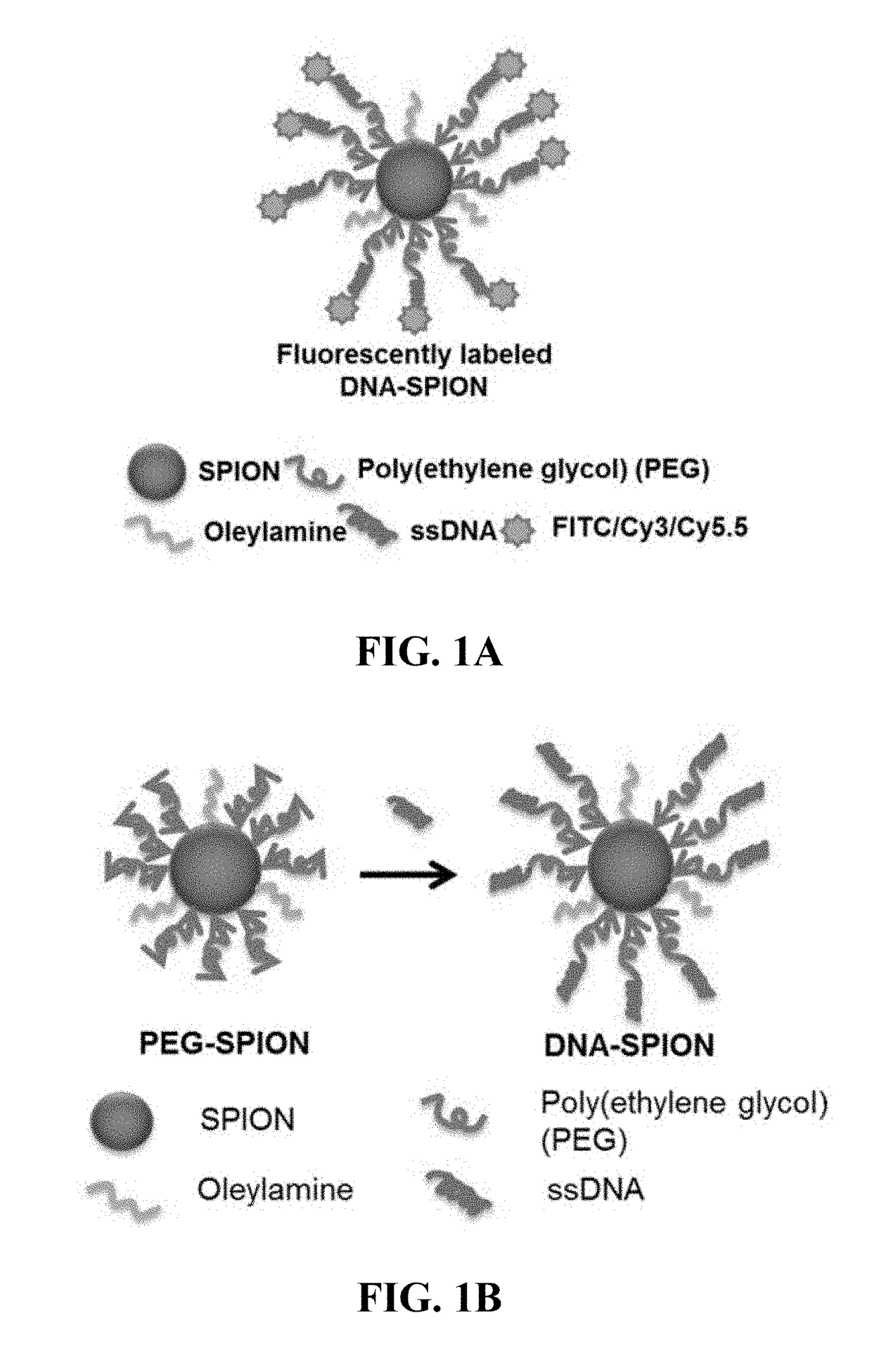
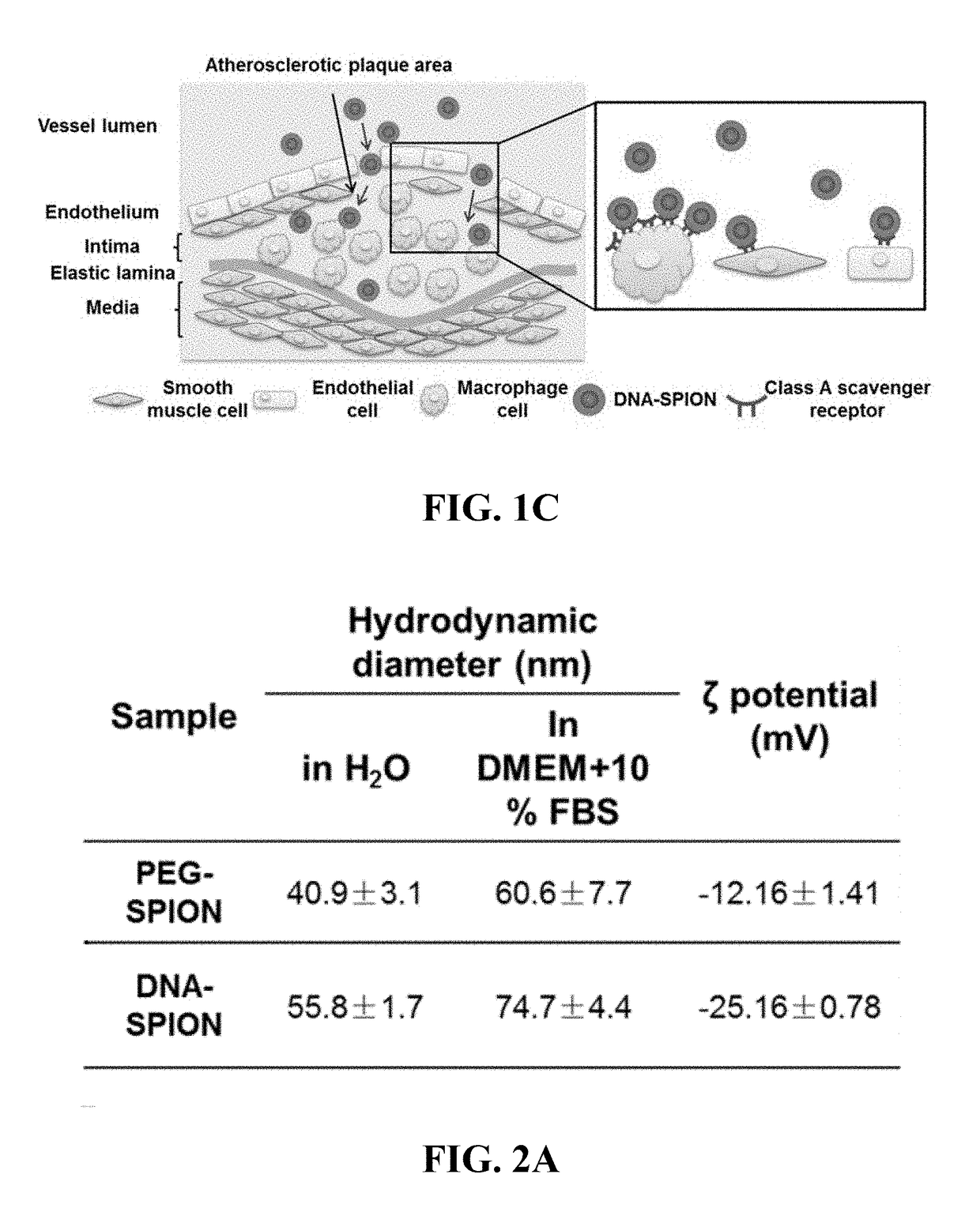
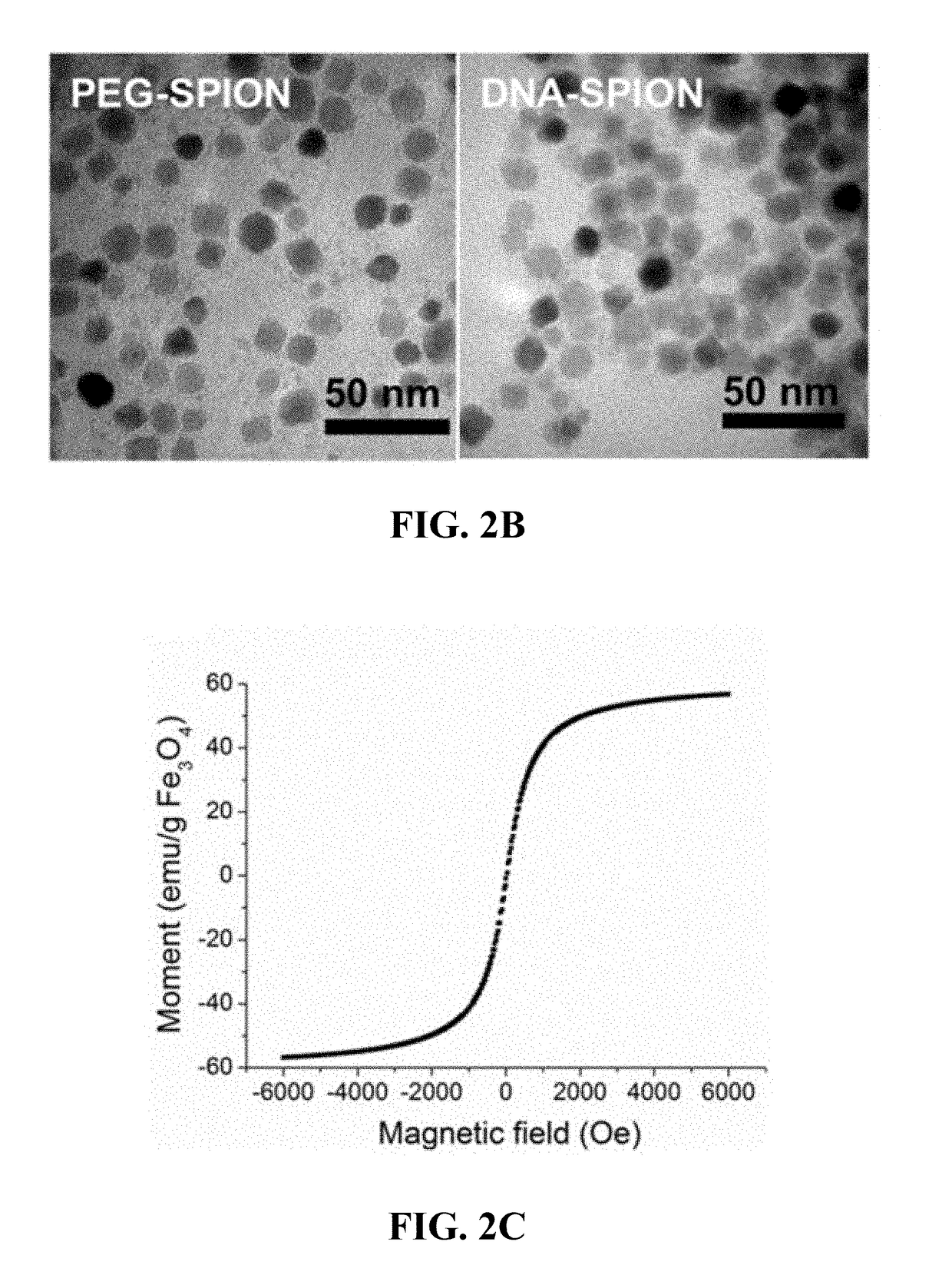
Materials and methods for effective in vivo delivery of DNA nanostructures to atherosclerotic plaques
ActiveUS20190060485A1Promote rapid accumulationPowder deliveryPharmaceutical non-active ingredientsSuperparamagnetic iron oxide nanoparticlesImaging agent
Provided are DNA-coated nanoparticles (DNA-NPS), superparamagnetic nanoparticles (DNA-SPNs), and superparamagnetic iron oxide nanoparticles (DNA-SPIONs) as efficient imaging agents for targeting and imaging atherosclerotic lesions and treating atherosclerotic disease. The DNA-NS, DNA-SPNs, and DNA-SPIONs can enter macrophage cells via the Class A scavenger receptor (SR-A)-mediated pathways and can be used to specifically target atheroscleortic plaques.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
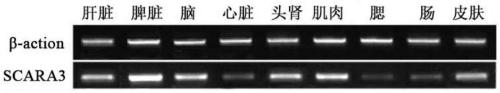
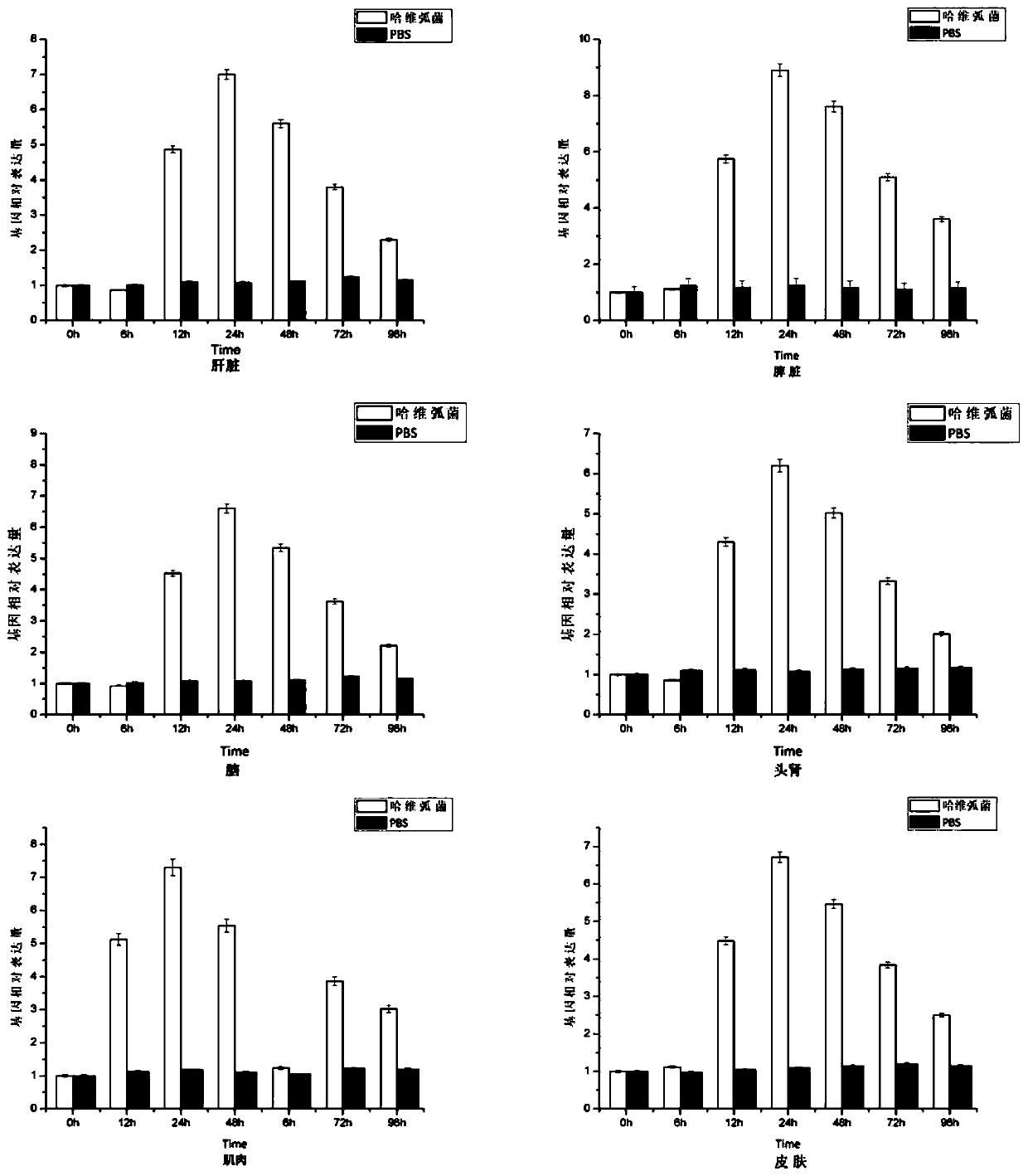
Large yellow croaker scavenger receptor SCARA3 gene and application thereof
PendingCN110358771AIdentify sensitiveRapid responseAntibacterial agentsPeptide/protein ingredientsAgricultural scienceFeed additive
The invention provides a large yellow croaker scavenger receptor SCARA3 gene and application thereof, and belongs to the technical field of genetic engineering. The complete ORF of the large yellow croaker scavenger receptor SCARA3 gene includes 1938 basic groups, and the nucleotide sequence is shown as SEQ ID NO:1; under the infection of vibrio harveyi, the expression quantity of the gene is up-regulated, and the gene can be applied to detecting the vibrio harveyi resistance of large yellow croaker parents and / or breeding the vibrio-harveyi-resistant fine species and especially can be appliedto detecting the vibrio harveyi resistance of the large yellow croaker parents and / or breeding the vibrio-harveyi-resistant fine species. Recombinant expression protein of large yellow croaker scavenger receptor protein coded by the large yellow croaker scavenger receptor SCARA3 gene has remarkable vibrio-harveyi-resistant activity and can be used for preparing vibrio-harveyi-resistant biologicalproducts, and a gene sequence and method are provided for the research on the large yellow croaker vibrio-harveyi-resistant mechanism and the development of vibrio-harveyi-resistant feed additives.
Owner:ZHEJIANG OCEAN UNIV
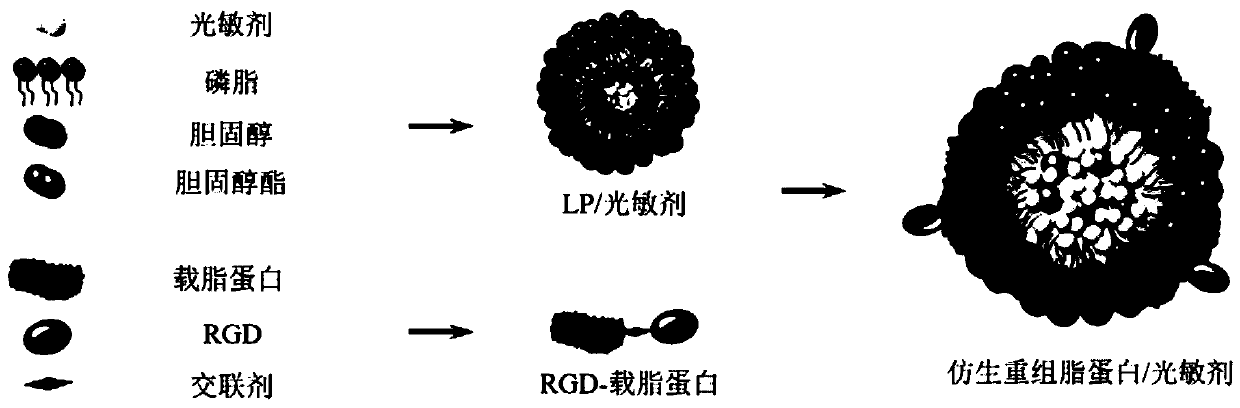
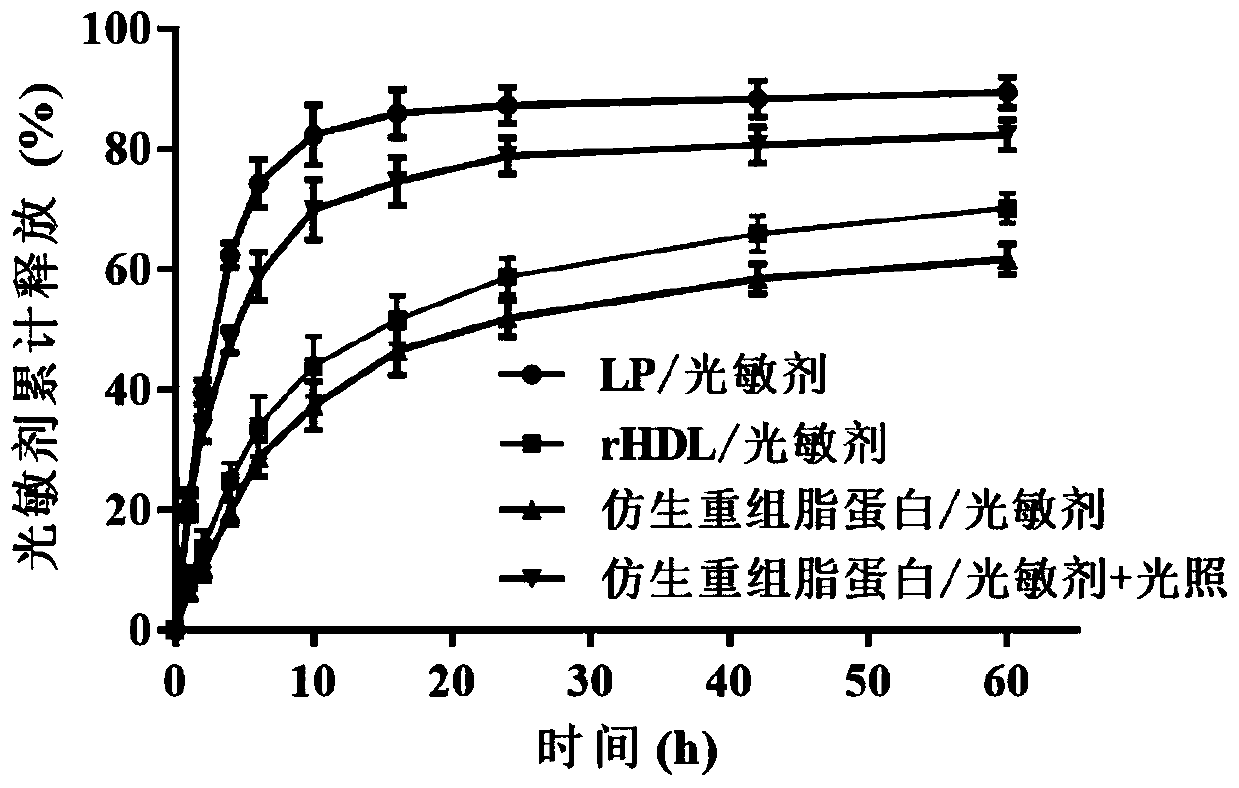
Bionic recombined lipoprotein/photosensitizer nanoparticle, and preparation method and application of bionic recombined lipoprotein/photosensitizer nanoparticle in diagnosis and treatment
InactiveCN110179978AQuick releaseAchieve therapeutic effectPowder deliveryPhotodynamic therapyArginineFluorescence
The invention discloses a bionic recombined lipoprotein / photosensitizer nanoparticle, and a preparation method and an application of the bionic recombined lipoprotein / photosensitizer nanoparticle. Thenanoparticle comprises a phospholipid monomolecular layer, and a photosensitizer and cholesteryl ester that are enveloped in the phospholipid monomolecular layer, wherein RGD (arginine-glycine-aspartic acid) peptide modified apolipoprotein is embedded into the surface of the phospholipid monomolecular layer; and cholesterol is further distributed among phospholipid molecules of the phospholipid monomolecular layer. The nanoparticle is effectively accumulated at a tumor site through high permeability and a retention effect of a solid tumor and high affinity of the apolipoprotein and a scavenger receptor, and further achieves target deep penetration to the tumor using high affinity of RGD and an integrin receptor. The nanoparticle can generate active oxygen and high heat under irradiation of near infrared light, promotes quick release of the photosensitizer from lipoprotein, and achieves a synergic photodynamic and photo-thermal treatment effect; and at the same time, fluorescent lightgenerated by triggering of near-infrared wavelength light can effectively perform in-vivo diagnosis, so that the nanoparticle achieves double functions of target treatment and diagnosis.
Owner:CHINA PHARM UNIV
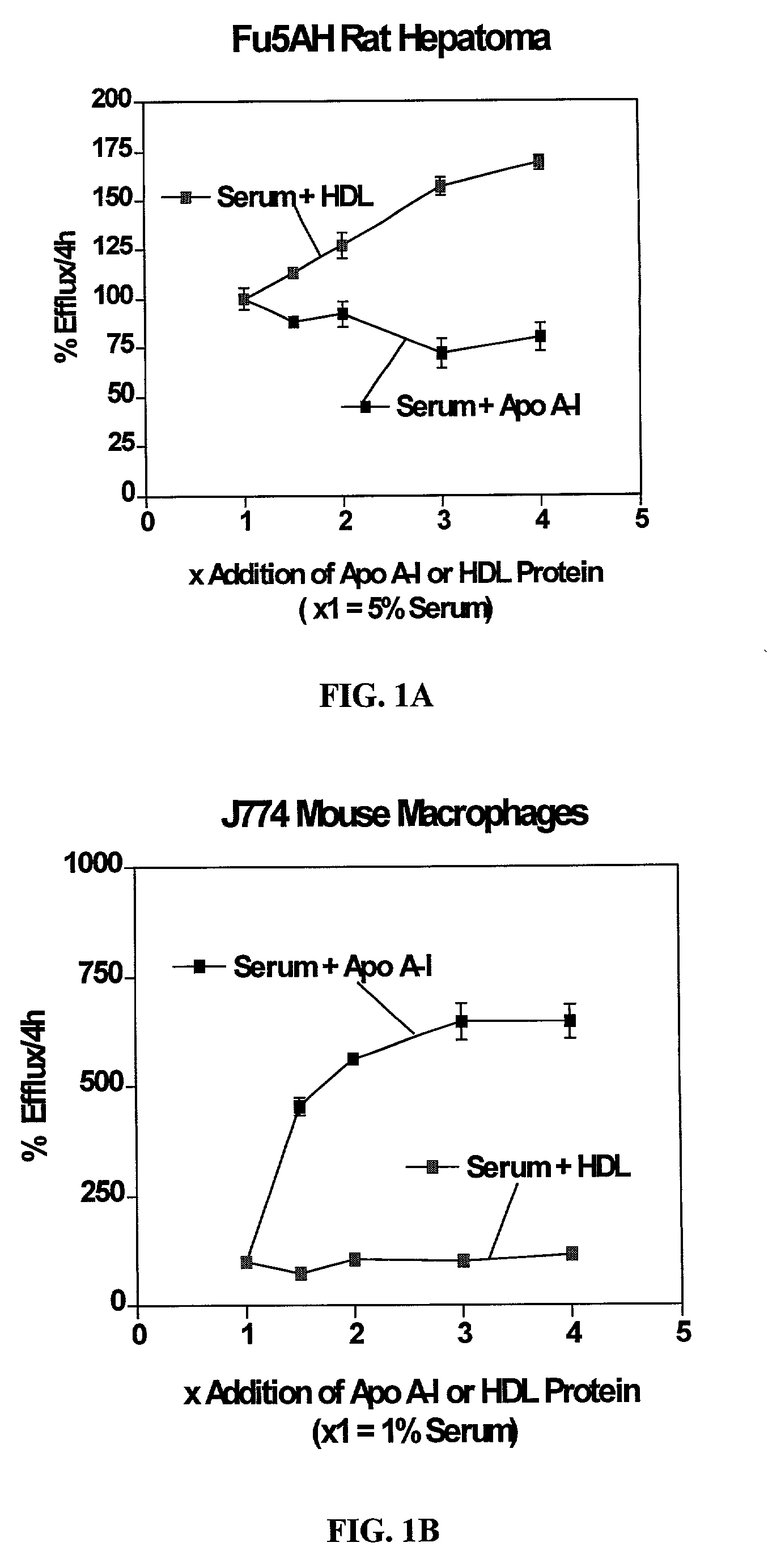
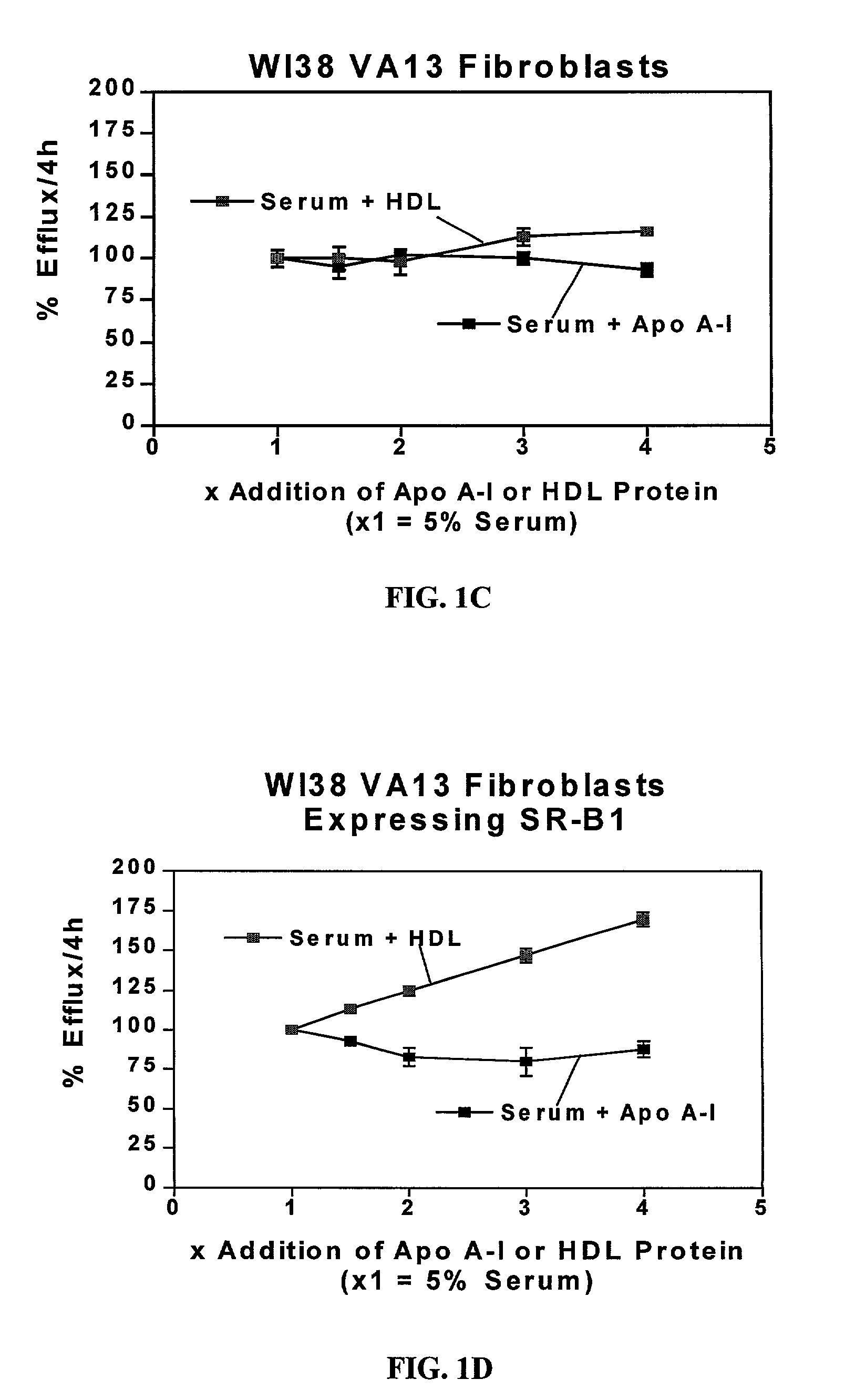
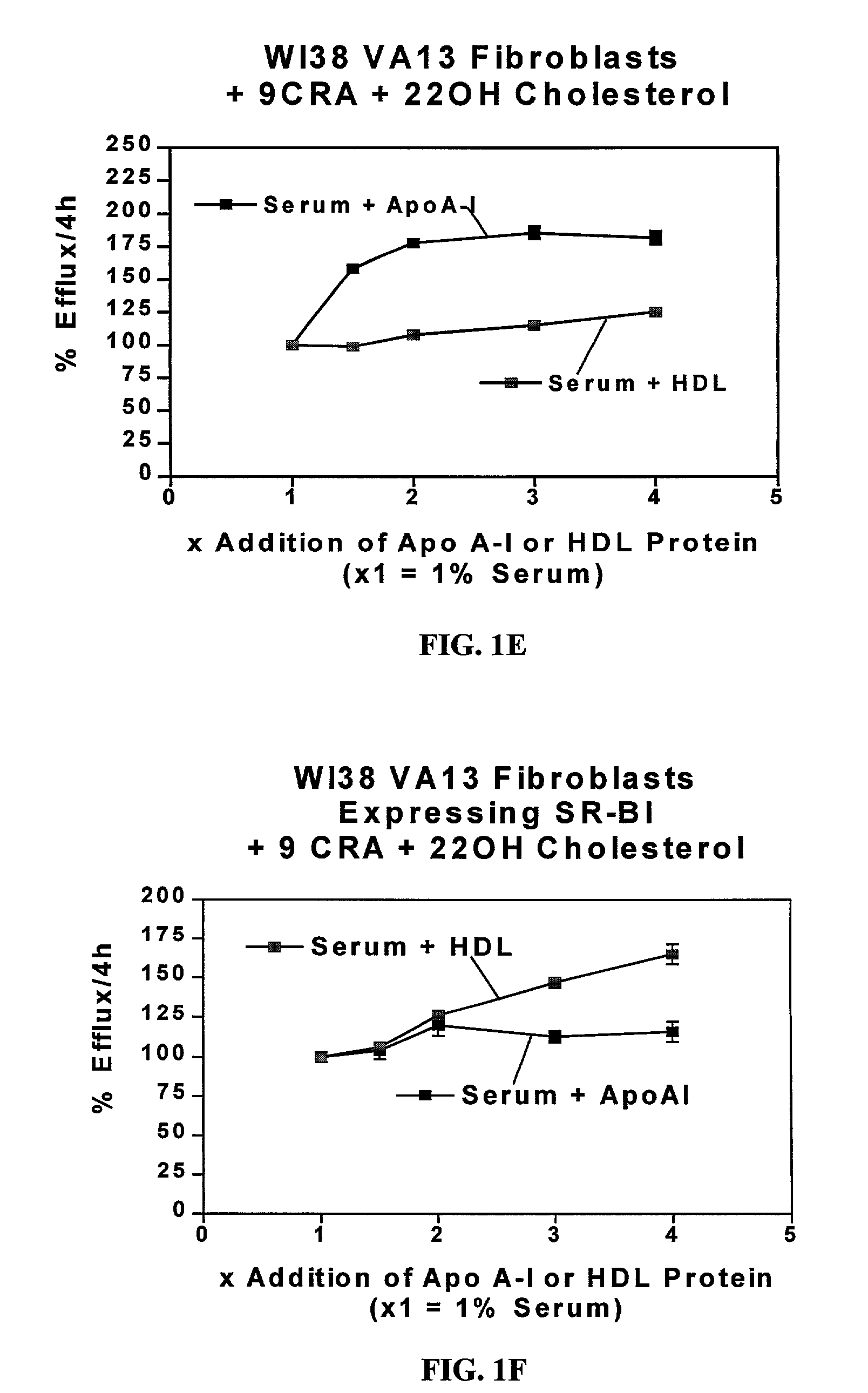
Cell culture system for determining the cholesterol efflux potential for serum
InactiveUS7029863B2Improve abilitiesEnhance serum efflux potentialMicrobiological testing/measurementDisease diagnosisSerum cholesterolScavenger receptor
The present invention relates to a cell culture system to provide a tool for assessing the potential of a patient's serum for preventing the accumulation of cholesterol in arteries (i.e. serum efflux potential) that leads to atherosclerosis and to screen new drug compositions being developed to reduce the accumulation of cholesterol or enhance the clearance of cholesterol from the vessel wall. The present invention combines two individual assays, which are two different cholesterol assays: an assay measuring scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux and an assay measuring ATP binding cassette protein 1 (ABCA1)-mediated cholesterol efflux, and uses them in parallel to test human and animal sera for their potential to stimulate efflux, as mediated by either of the two receptors described above.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Mouse model of chronic heart failure and coronary atherosclerosis regression
ActiveUS7960606B2Restore blood flowReduced activityCompounds screening/testingCell receptors/surface-antigens/surface-determinantsScavenger receptorLower blood cholesterol
An animal model has been developed where the animals can survive myocardial infarctions caused by diet-induced coronary atherosclerosis, and live with chronic heart failure. This animal model is a result of reduced activity of scavenger receptor class BI (SR-BI) and ApoE and the inducible activity of the Mx1-Cre gene. In a preferred embodiment, the model is a result of crossbreeding two transgenic mouse lines: a knockout of SR-BI (SRBI− / −) and an impaired ApoE expressor (Apoeh / h) to generate a strain referred to as Apoeh / hSRB1− / − mice, which is then crossbred to mice that carry the inducible Mx1-Cre transgene. The Apoeh / hSRB1− / − mouse model is genetically modified, enabling the offspring to rapidly and permanently lower their high blood cholesterol levels caused by dietary challenge. The ability to rapidly and permanently lower blood cholesterol levels in these mice stops and may cause the regression of occlusive coronary atherosclerosis restoring blood flow to the heart, allowing the mice to survive from myocardial infarction and live with chronic heart failure.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +2
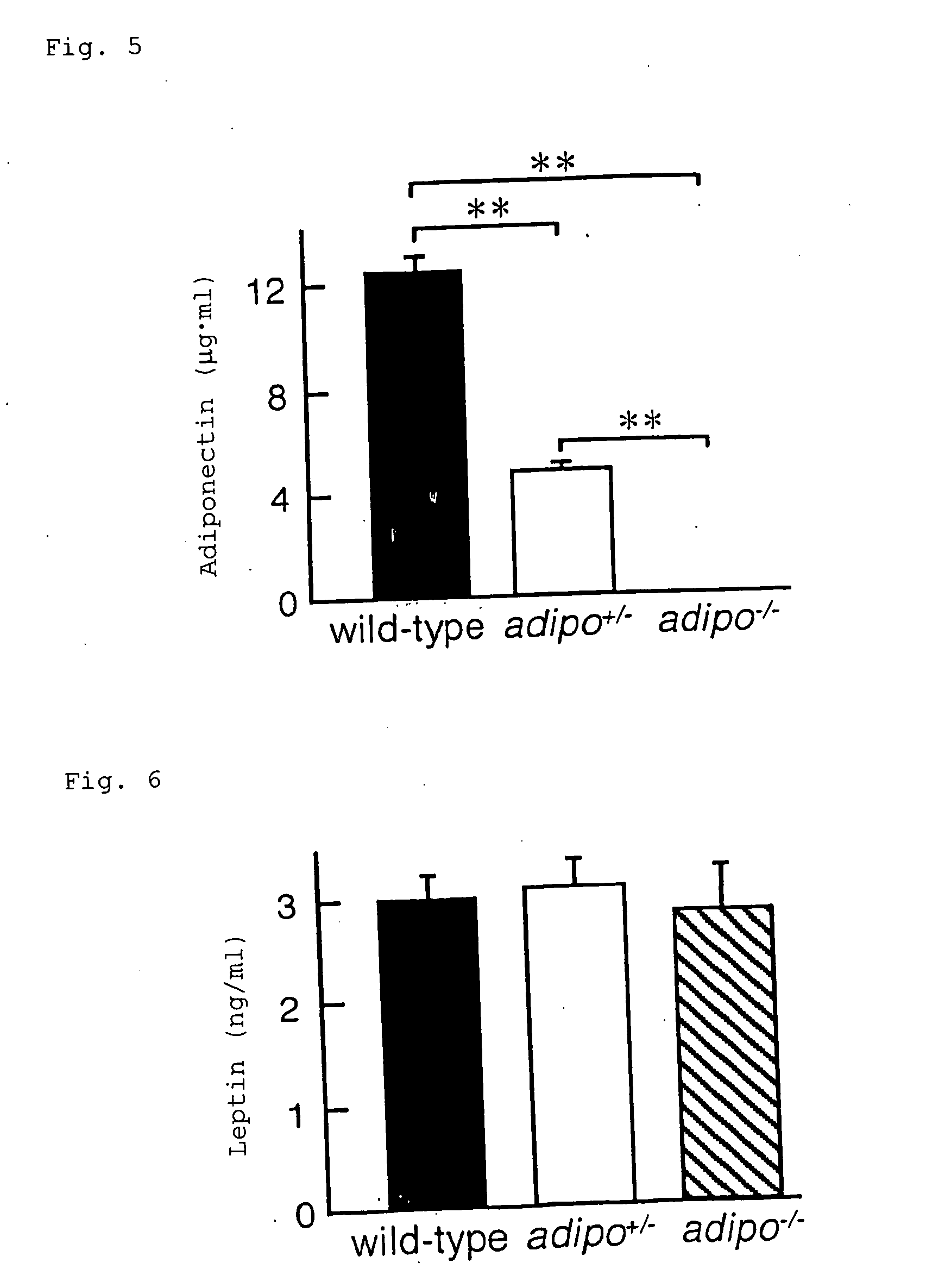
Preventive/remedy for arteriosclerosis
InactiveUS20060166873A1Thickened arterialReduced expression levelPeptide/protein ingredientsMetabolism disorderLipid formationBULK ACTIVE INGREDIENT
A scavenger receptor A expression down-regulator and a drug for preventing or treating arteriosclerosis which contain, as the active ingredient, a C-terminal globular domain of adiponectin, adiponectin, or a gene encoding the domain or adiponectin. According to the present invention, there is provided a preventive or therapeutic agent capable of directly preventing intimal thickening, which constitutes an essential feature of arteriosclerosis. This effect can be attained through arresting the onset and development of arteriosclerosis by reducing the expression level of scavenger receptor A in arterial walls and preventing lipid buildup in macrophages.
Owner:JAPAN SCI & TECH CORP
Application of SR-A as diagnostic marker and intervention target for rheumatoid arthritis
ActiveCN106526196AGood diagnostic specificityImprove performanceDisease diagnosisBiological testingScavenger receptorDisease course
The present invention discloses application of an A-type scavenger receptor (SR-A) as a diagnostic marker and an intervention target for rheumatoid arthritis, the accuracy rate of an A-type scavenger receptor (SR-A) protein as a marker for clinical diagnosis of the rheumatoid arthritis reaches to 90%, and compared with the accuracy rate of other diagnostic markers, the detection accuracy is greatly improved. In the aspect of treatment of the rheumatoid arthritis (RA), the SR-A as a therapeutic target also shows excellent performance, a SR-A neutralizing antibody can effectively prevent development of RA disease, and especially shows a very good therapeutic effect on disabling RA bone destruction.
Owner:PEOPLES HOSPITAL PEKING UNIV
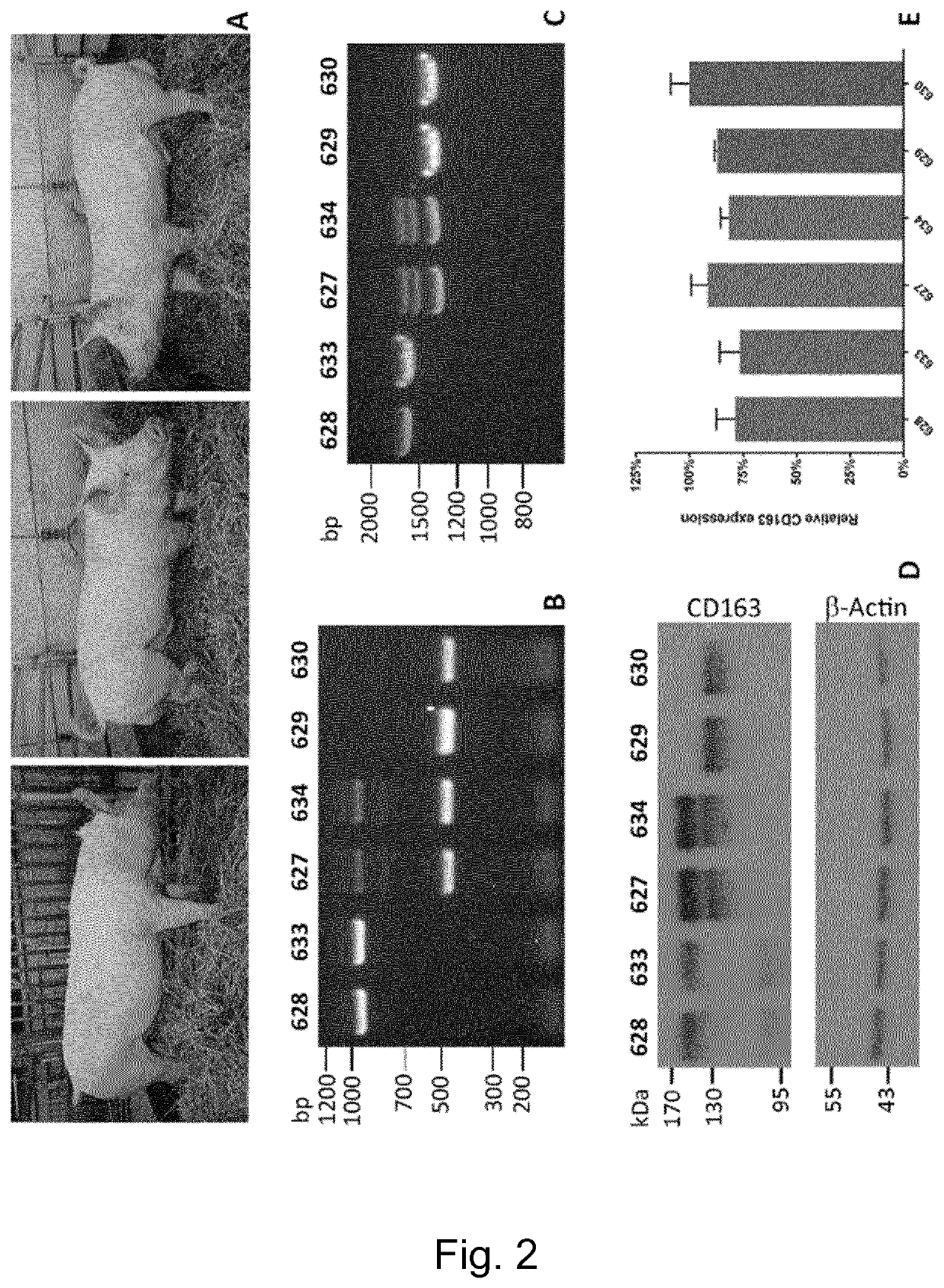
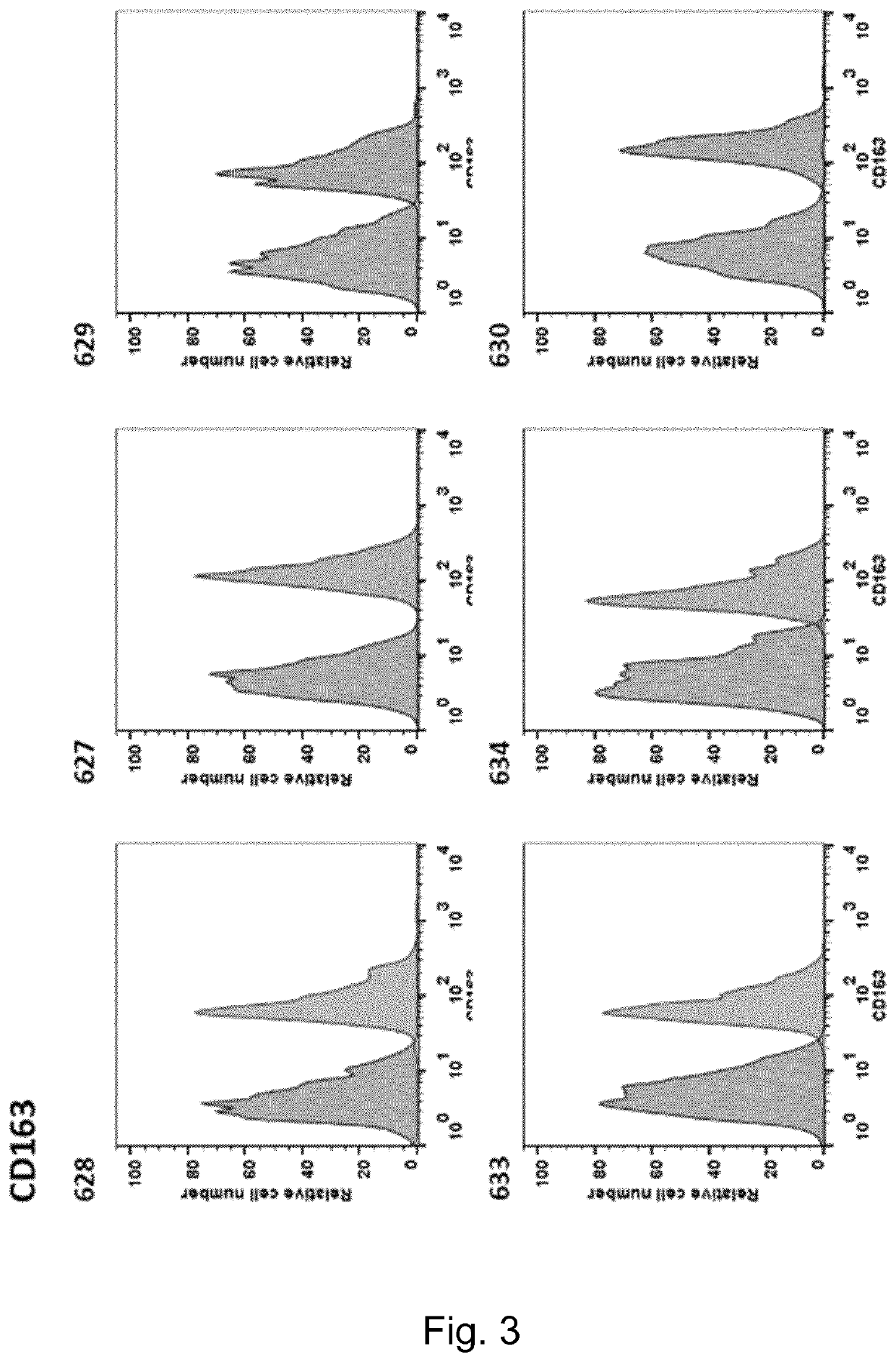
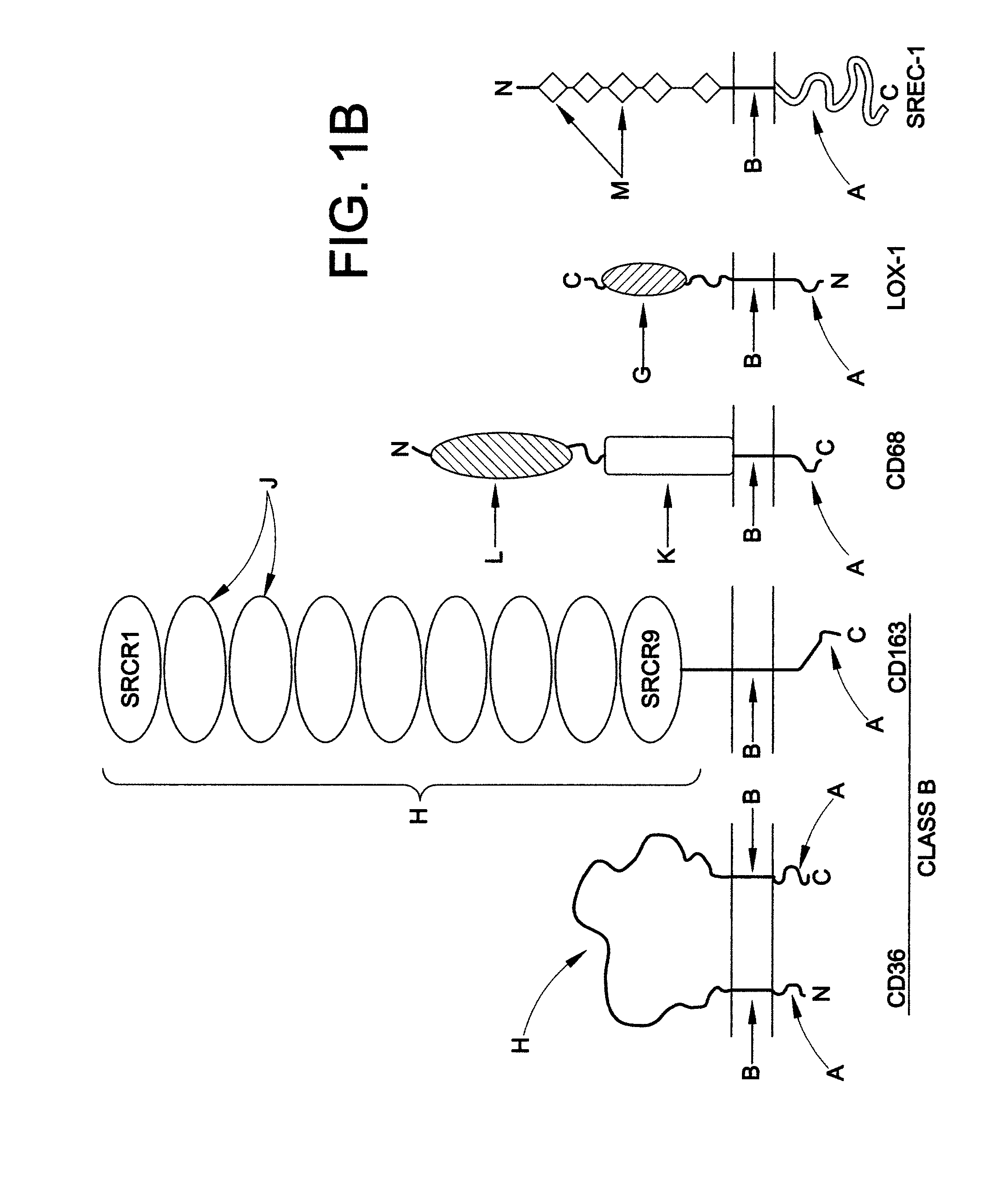
Swine Comprising Modified CD163 and Associated Methods
InactiveUS20200045945A1Cell receptors/surface-antigens/surface-determinantsGenetically modified cellsScavenger receptorCoboglobin
The present invention relates to genetically edited swine which produce CD163 protein in which the scavenger receptor cysteine-rich 5 (SRCR5) domain (also known as CD163 domain 5) has been deleted. Such swine have been found to be healthy and do not exhibit negative properties, and are resistant to PRRSV infection. CD163 expressed in the edited swine also demonstrates retention of the ability to function as a haemoglobin-haptoglobin scavenger. Methods of producing such swine are also provided.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
Inducible heart attack animal model
InactiveUS7514592B2Compounds screening/testingCell receptors/surface-antigens/surface-determinantsApolipoprotein activityRAG2
An animal model of coronary heart disease has been developed where myocardial infarct can be induced by altering the animal's diet. In all embodiments, this animal model is a result of reduced activity of scavenger receptor class BI (SR-BI) and apolipoprotein E (ApoE). In a preferred embodiment, the model is a result of crossbreeding two transgenic mouse lines: a knockout of SR-BI (SR-BI− / −) and an impaired ApoE expressor (hypoE). The impaired ApoE gene results in only 2-5% expression of ApoE and a reduction in cholesterol homeostasis. Resulting animals are predisposed to hypercholesterolemia but can live longer than a year on a normal low fat diet. Serum plasma levels can be significantly elevated by changing the animal's diet to one containing high levels of fat and cholesterol. Within a month on a high fat, high cholesterol diet, animals develop atherosclerosis and myocardial infarction occurs. Survival depends on the nature of the diet and the conditions of animal husbandry and can typically be around 20-30 days after administration of the modified diet depending on the specific conditions. Housing the animals alone or in groups significantly affects survival of these animals on a high fat diet. Analysis of B- and T-cell deficient SR-BI / ApoE / RAG2 triple knockout mice established that B- and T-lymphocytes do not play a key role in the pathophysiology of the SR-BI ApoE dKO model of human disease. These animal models can be used to study mechanisms and progression of CHD as a function of diet, treatment with drugs to be screened for efficacy or undesirable side effects, and social environmental effects.
Owner:MASSACHUSETTS INST OF TECH
Yeast recombinant expression, purification and application of protein functional polypeptide with effect of preventing and treating atherosclerosis
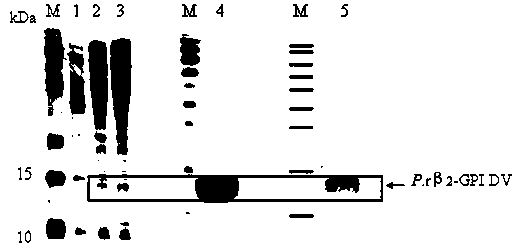
ActiveCN104109686APreempt the binding siteFully foldedMicroorganism based processesPeptide preparation methodsBio engineeringRecombinant expression
The invention belongs to the field of biological engineering, and in particular to recombinant expression and purification of a fifth functional domain of an oxLDL binding protein human serum beta 2-glycoprotein I and the application thereof to diagnosis prevention and treatment of atherosclerosis. A designed primers pair is subjected to PCR amplification on a T vector of a beta 2-GPI gene fragment to obtain a gene fragment of a beta 2-GPI fifth functional domain; and pPICZ alpha A-DV is constructed by cloning, and is then subjected to power transformation to obtain a positive strain, which is subjected to recombination and purification to obtain a P.r beta 2-GPI-DV protein. The possibility of using the recombinant protein as a drug in the prevention and treatment of atherosclerosis and the feasibility of detecting the oxLDL content in serum are proved. The P.r beta 2-GPI-DV recombinant protein has purity above 90%, can reduce the content of oxLDL / beta 2-GPI binary compound in the serum, and then inhibit the uptake of oxLDL by scavenger receptors on the surface of macrophage, so as to achieve the effect of preventing and treating atherosclerosis; and because of the combination specificity with oxLDL, the P.r beta2-GPI-DV can realize the specific detection of oxLDL in serum.
Owner:DALIAN UNIV
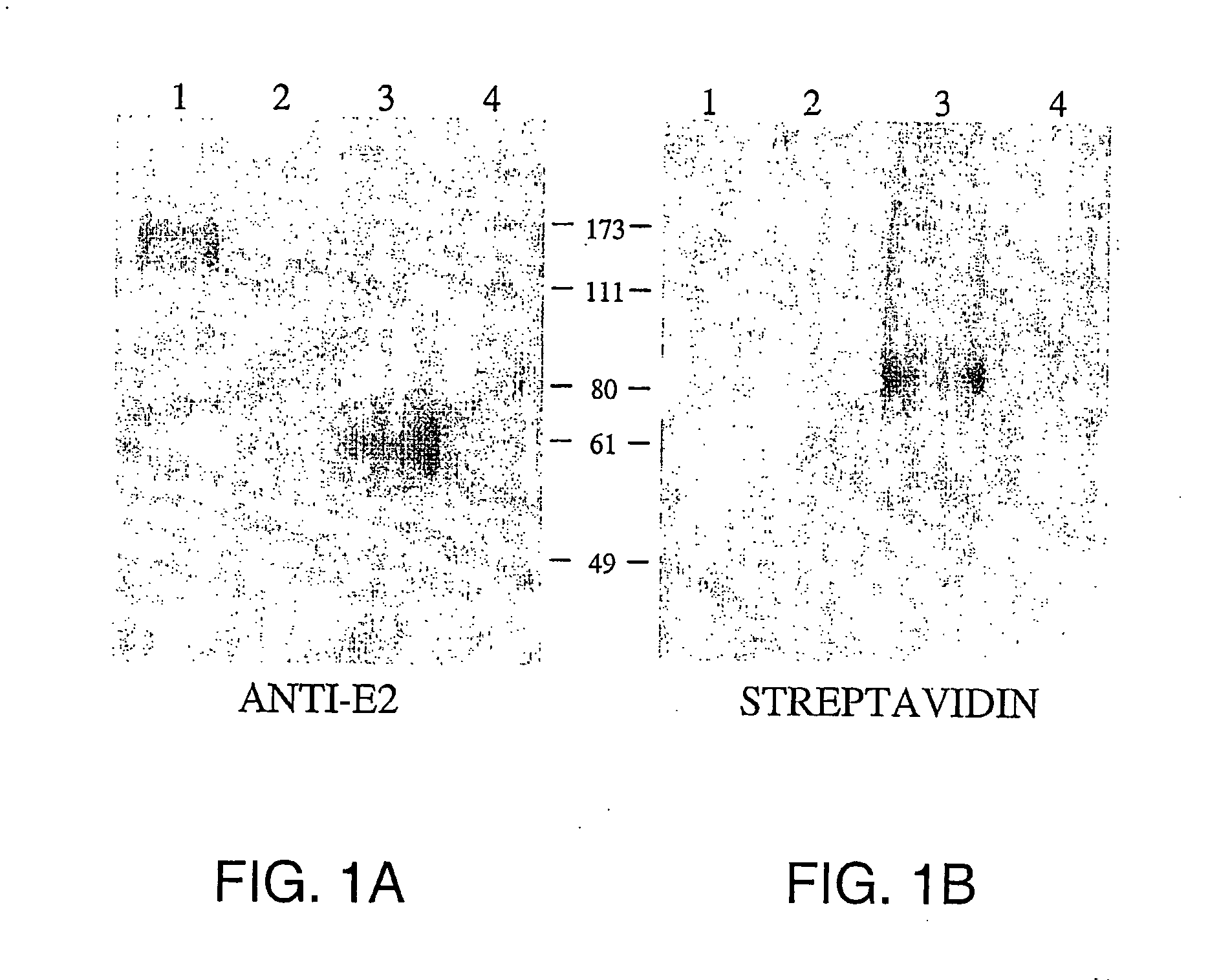
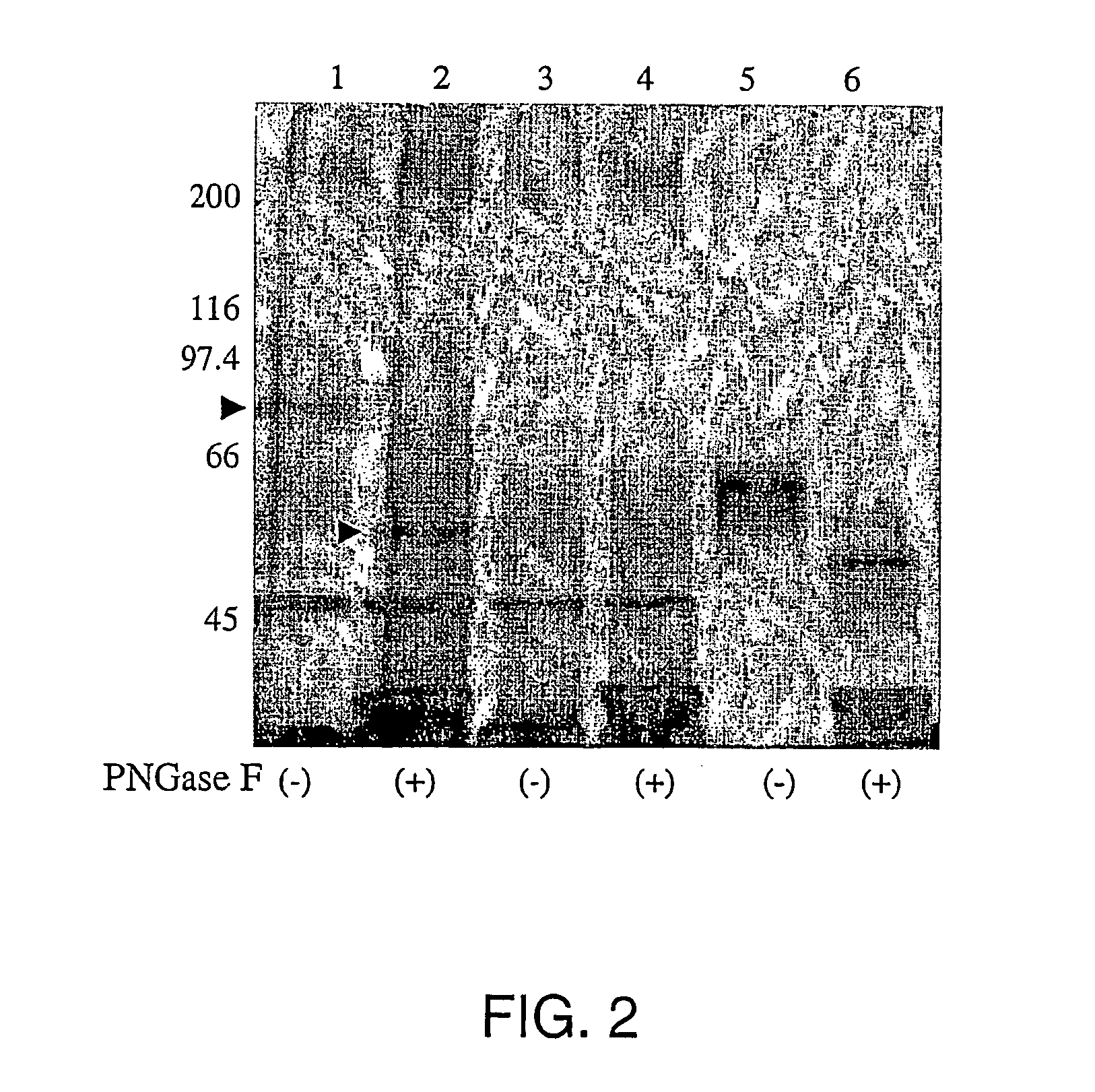
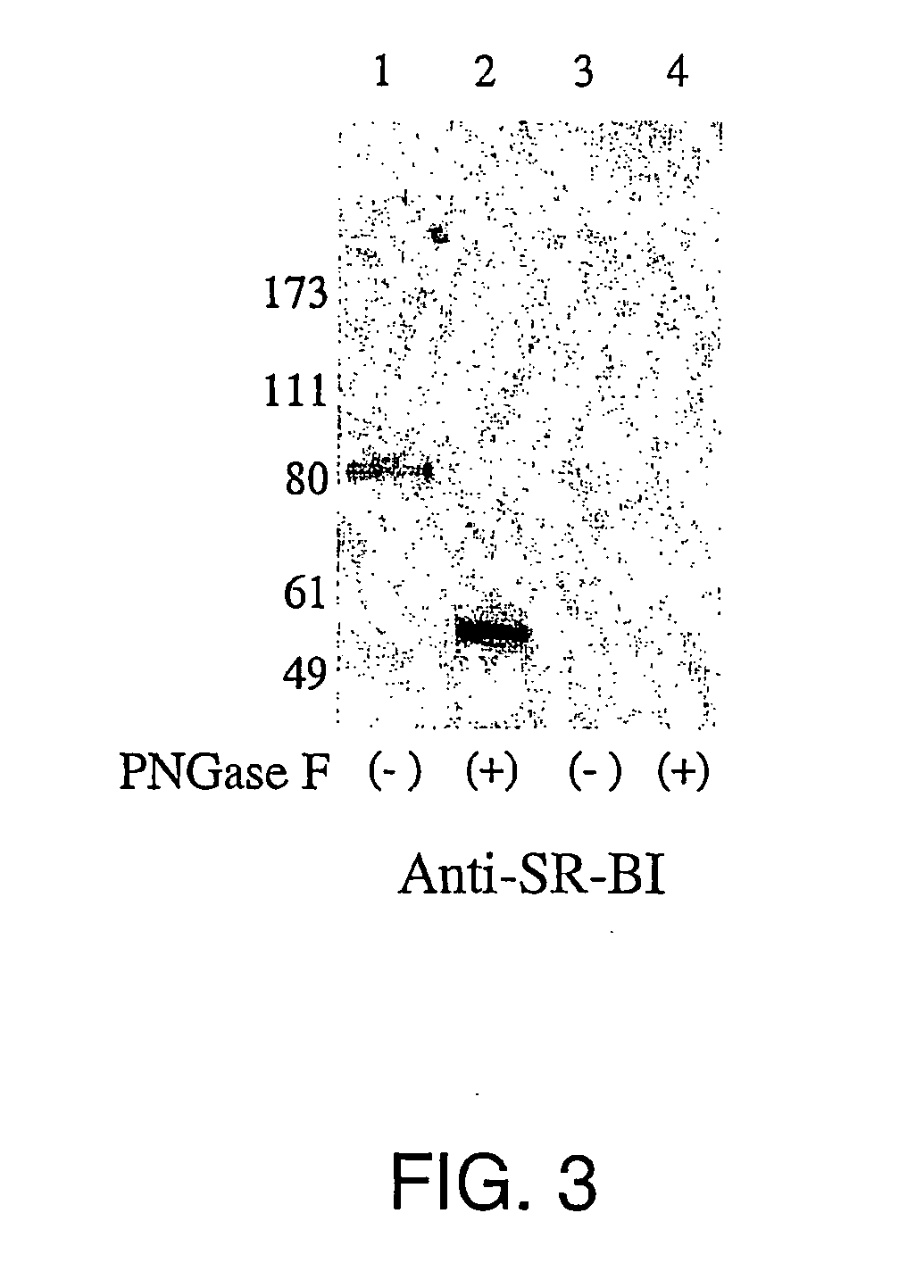
Screening for hepatitis c virus entry inhibitors
InactiveUS20050019751A1Inhibit bindingDecreasing functional surface expressionCompound screeningApoptosis detectionScavenger receptorProphylactic treatment
The present invention features methods of screening for compounds that inhibit HCV binding to a cell, methods of inhibiting IICV entry into a cell, and methods of actively or prophylactically treating against an IICV infection. The different methods are based on the identification of the scavenger receptor class B type I as a target site for HCV E2 binding to a cell.
Owner:IST DI RICERCHE DI BIOLOGIA MOLECOLARE P ANGELETTI
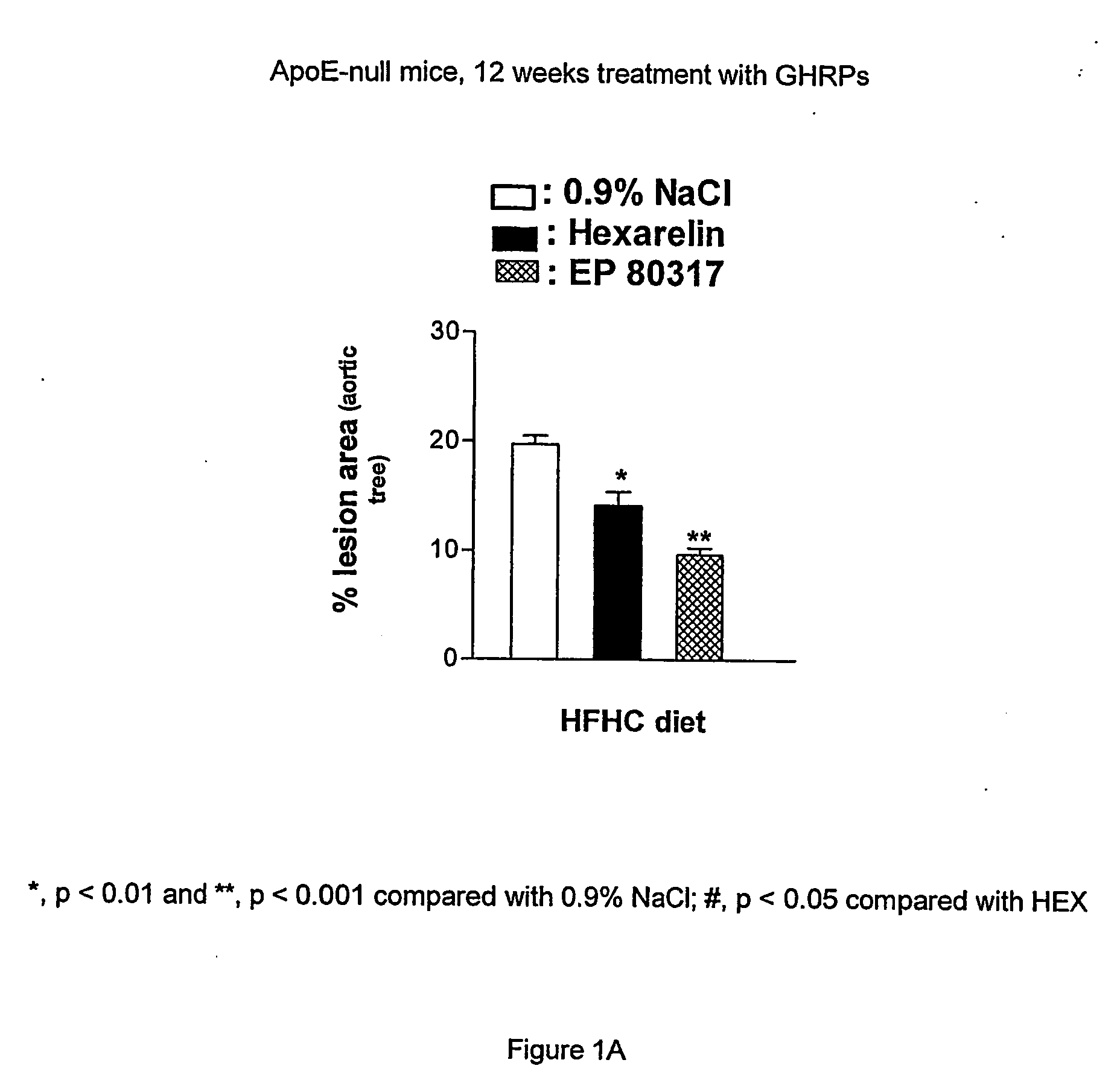
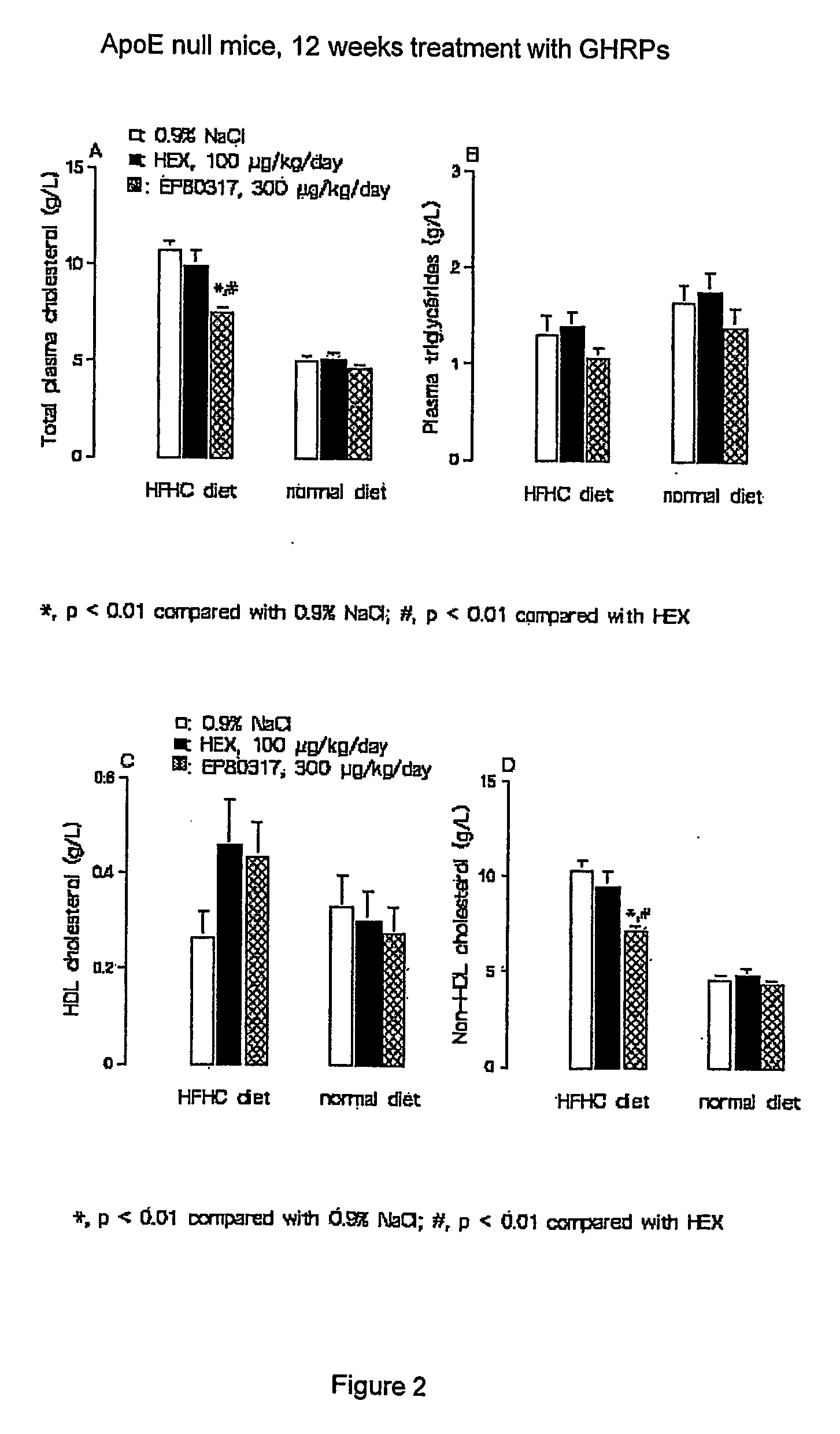
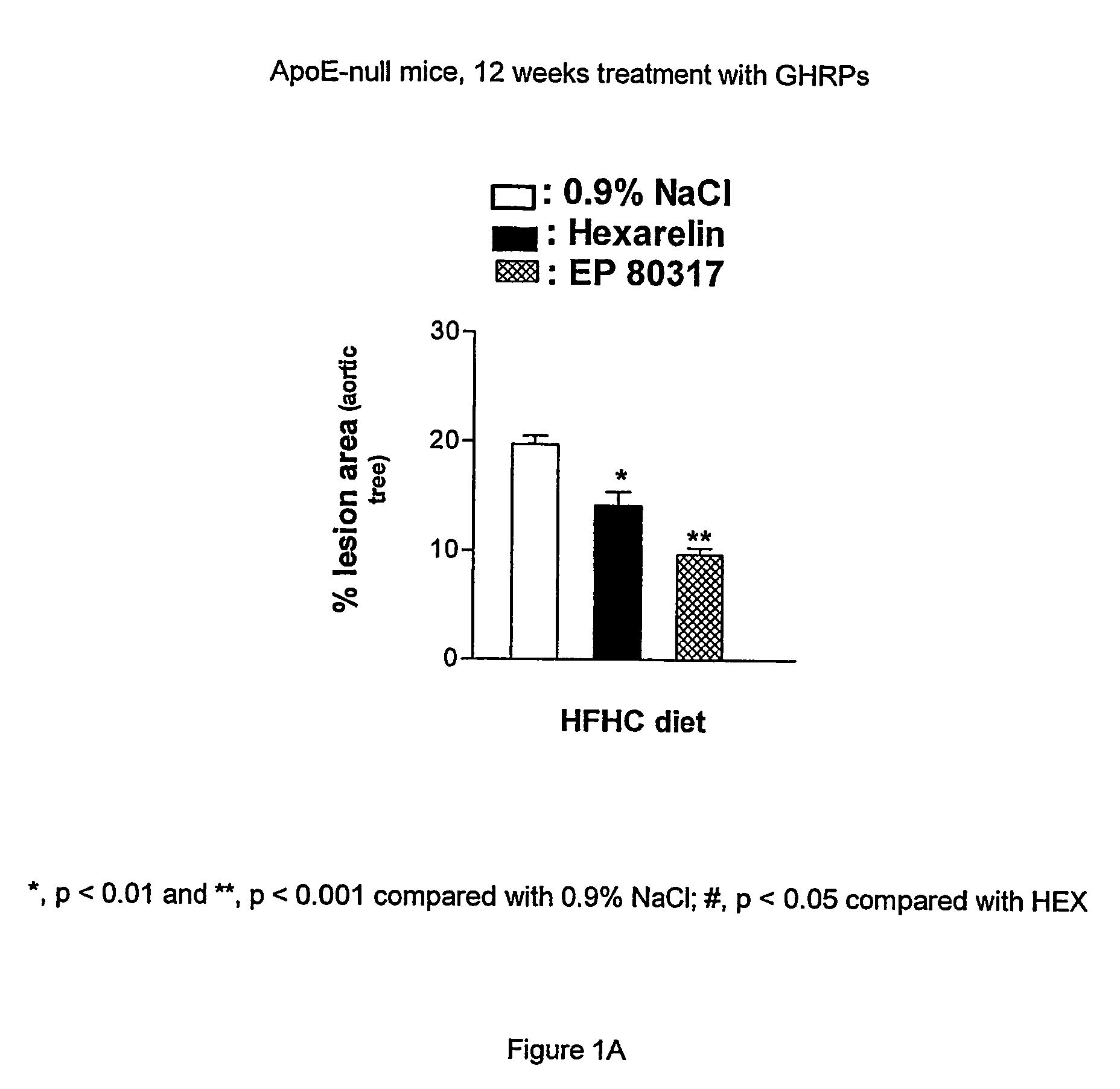
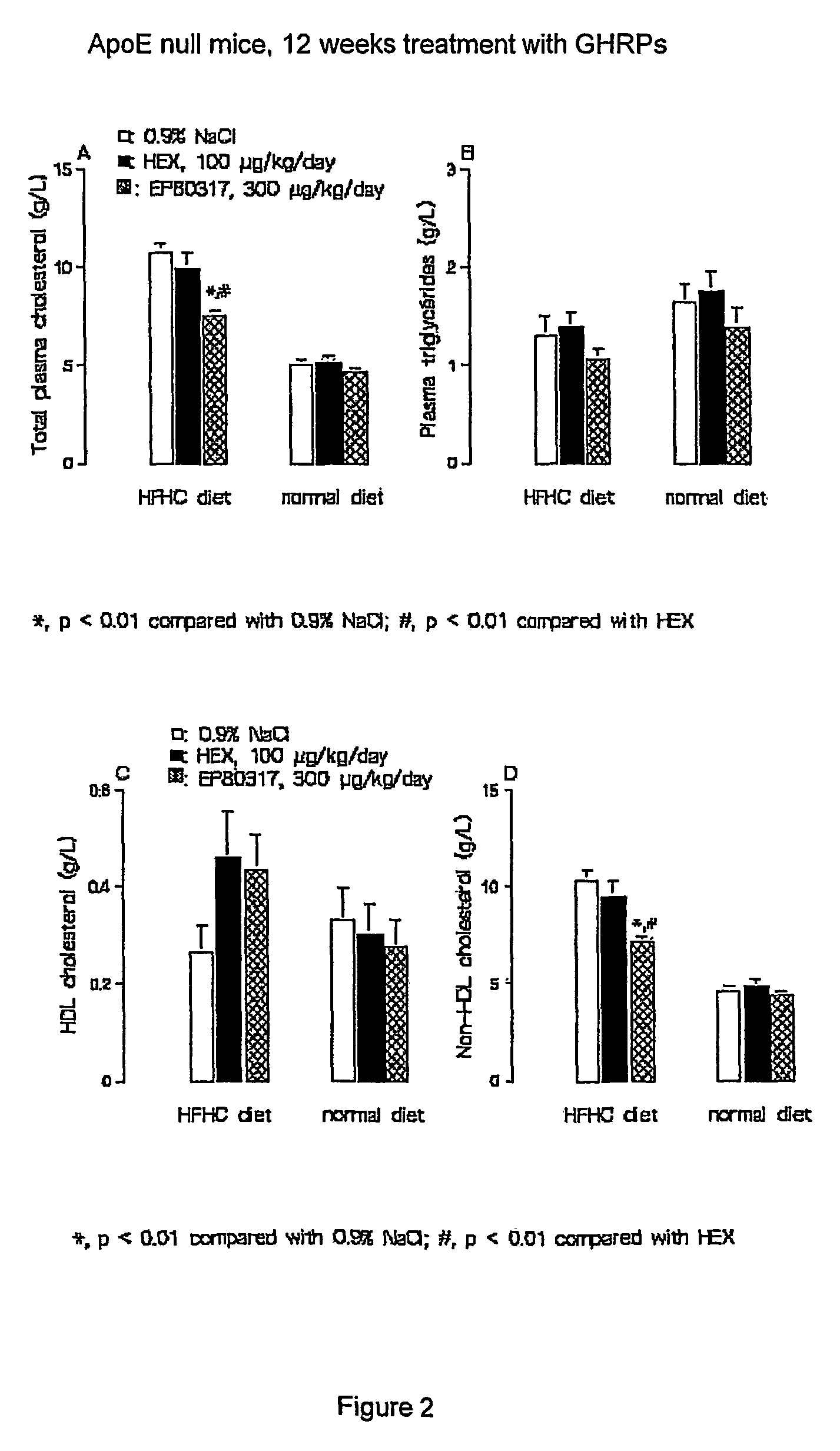
Growth hormone-releasing peptides in the treatment of prevention of atherosclerosis and hypercholesterolemia
InactiveUS20060241054A1Reduce lipid accumulationReduce lesionsPeptide/protein ingredientsMetabolism disorderScavenger receptorCellular cholesterol
According to the invention there is provided a method of treatment or prophylaxis of atherosclerosis, hypercholesterolemia or a cardiovascular disease associated with atherosclerosis, which method comprises administration of one or more Growth Hormone Releasing Peptides (GHRPs) to a patient in need of such treatment or prophylaxis. There are also provided methods of reducing blood plasma cholesterol levels, as well as methods of modulating the expression of the scavenger receptor CD36 and genes involved in cellular cholesterol efflux.
Owner:VALORISATION HSJ SOC & COMMANDITE CO CHU SAINTE JUSTINE LE CENT HOSPITALIER UNIV MERE ENFANT
Blood cell production via activation of the hemoglobin scavenger receptor
The present invention describes methods and compositions for stimulating the growth, proliferation, differentiation and / or mobilization of stem cells and / or progenitor cells. The method involves administering an effective amount of a substance that activates a CD163 hemoglobin scavenger receptor signaling pathway. The methods and compositions are useful for stimulating hematopoiesis and treating a variety of disorders, including cytopenias, anemia, and for preparing cells for transplantation.
Owner:赫姆索尔有限合伙公司
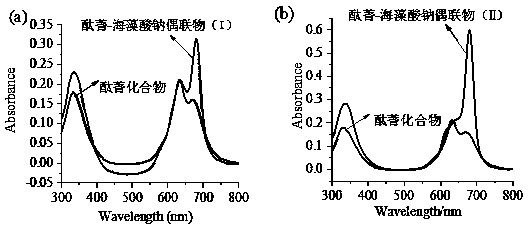
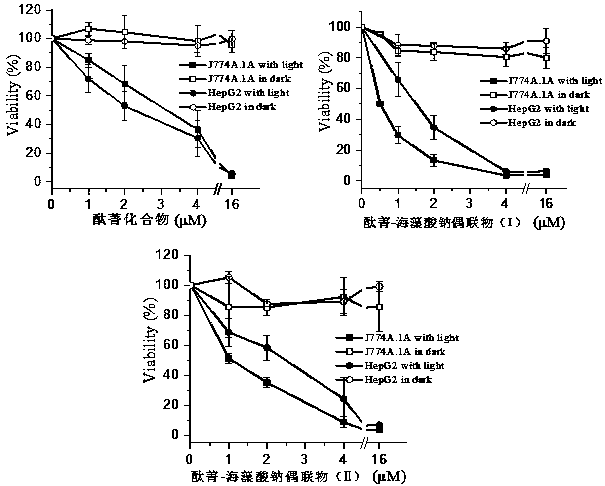
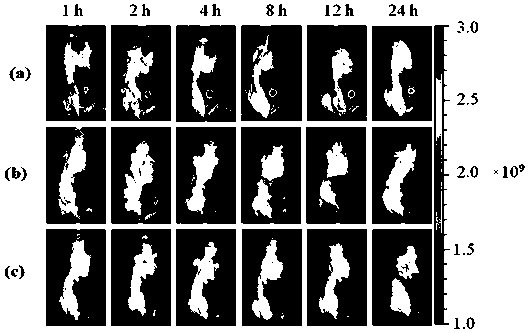
Application of sodium alginate as antitumor drug targeting carrier
ActiveCN111012911ARich sourcesLow priceAntibacterial agentsEnergy modified materialsTumor targetingPhthalocyanine
The invention discloses an application of sodium alginate as an antitumor drug targeting carrier and a phthalocyanine-sodium alginate conjugate prepared based on the application. According to the invention, the characteristic that a scavenger receptor on the surface of tumor tissue related macrophages has selectivity on sodium alginate is utilized; sodium alginate is used as a carrier; the phthalocyanine-sodium alginate conjugate is generated by coupling a carboxyl group on sodium alginate and amino in a phthalocyanine compound through an amido bond. The conjugate not only has high photosensitive activity and excellent water solubility and biocompatibility, but also has good tumor targeting and can be applied to photodynamic anti-cancer and photodynamic diagnosis due to the fact that sodium alginate can enter cells through scavenger receptors A on the surfaces of tumor-associated macrophages to achieve enrichment of sodium alginate in tumor tissues.
Owner:FUZHOU UNIV
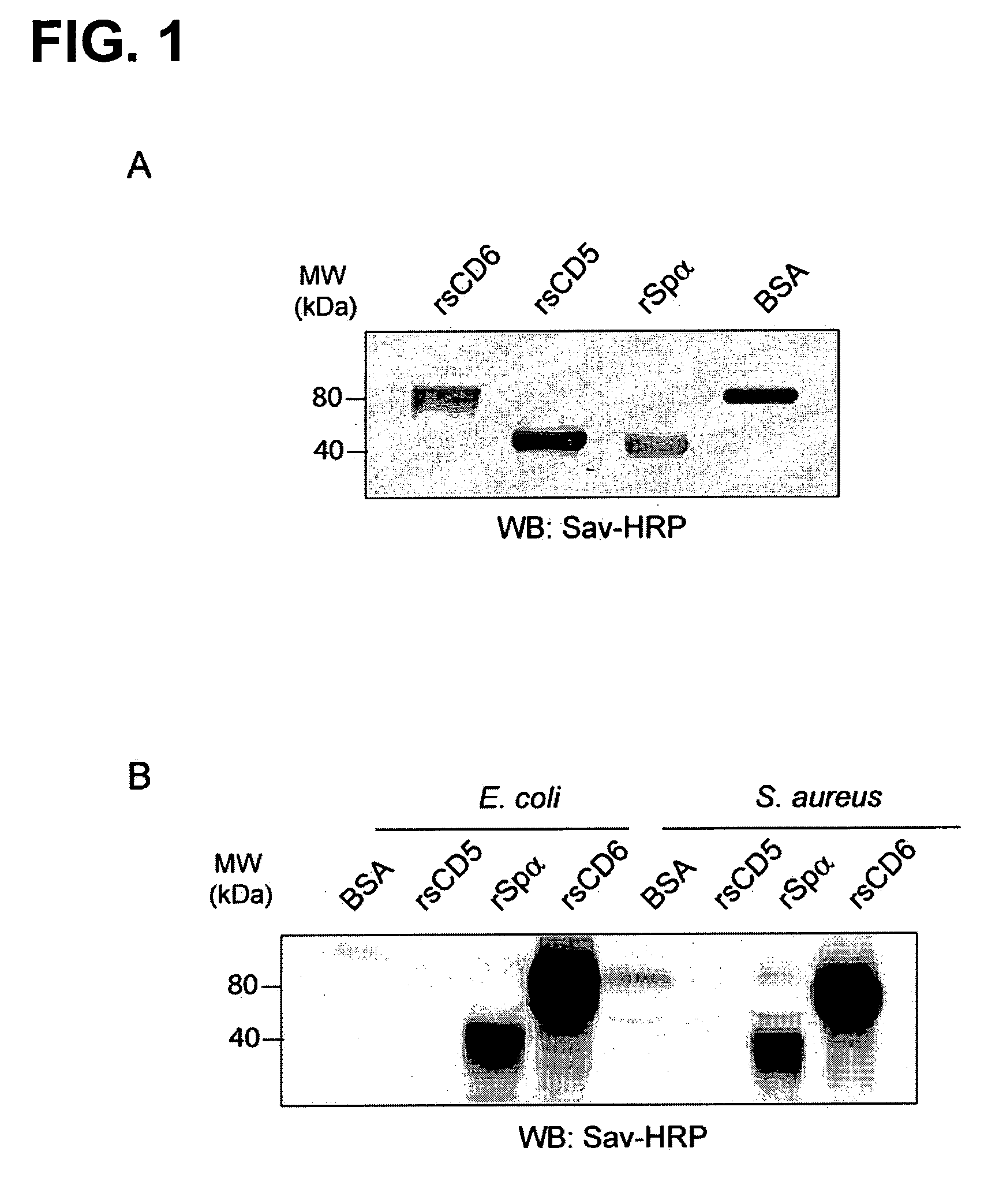
Protein Product for Treatment of Infectious Diseases and Related Inflammatory Processes
ActiveUS20100105622A1High affinityIncrease in sizeAntibacterial agentsAntimycoticsInflammation ProcessInfective disorder
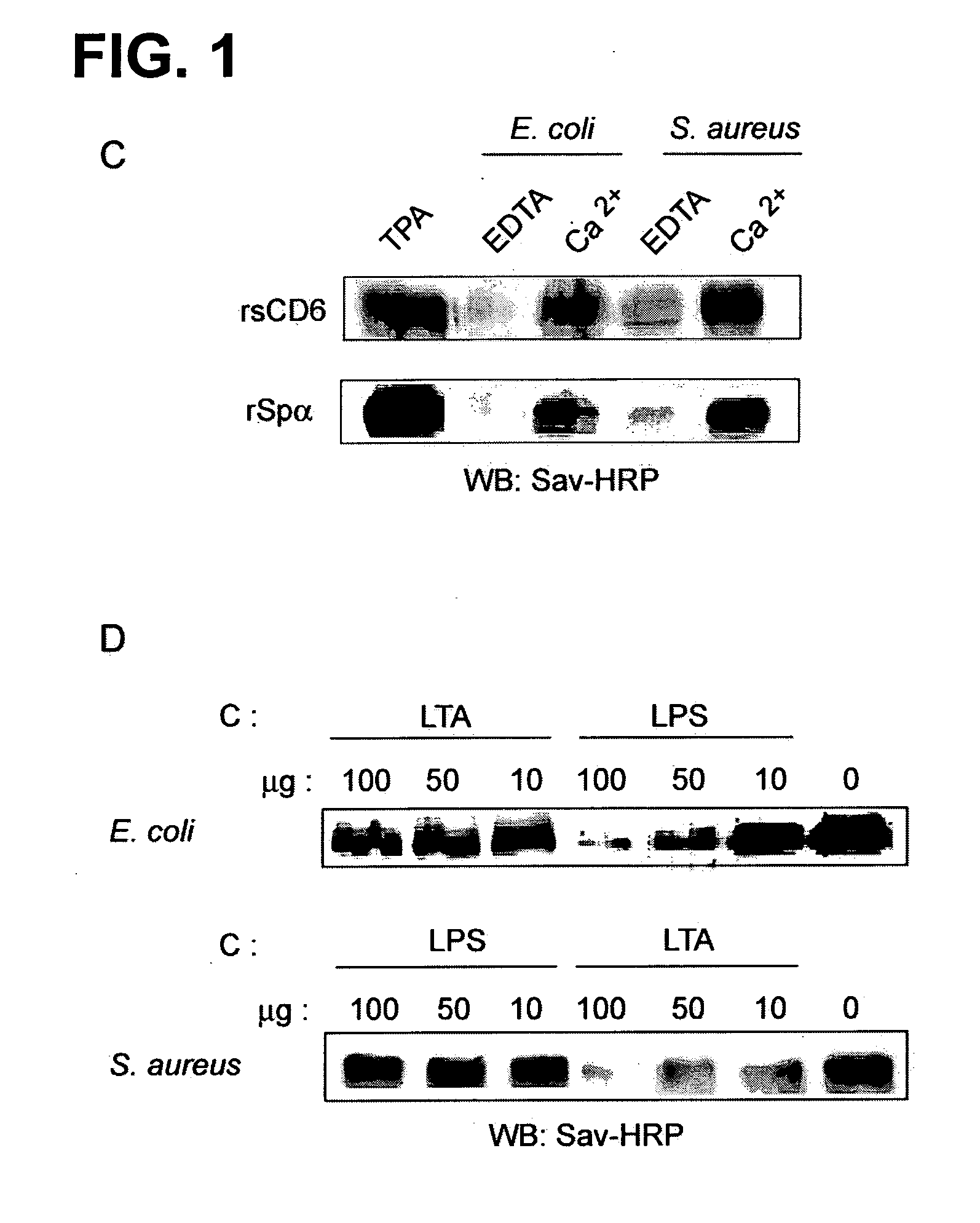
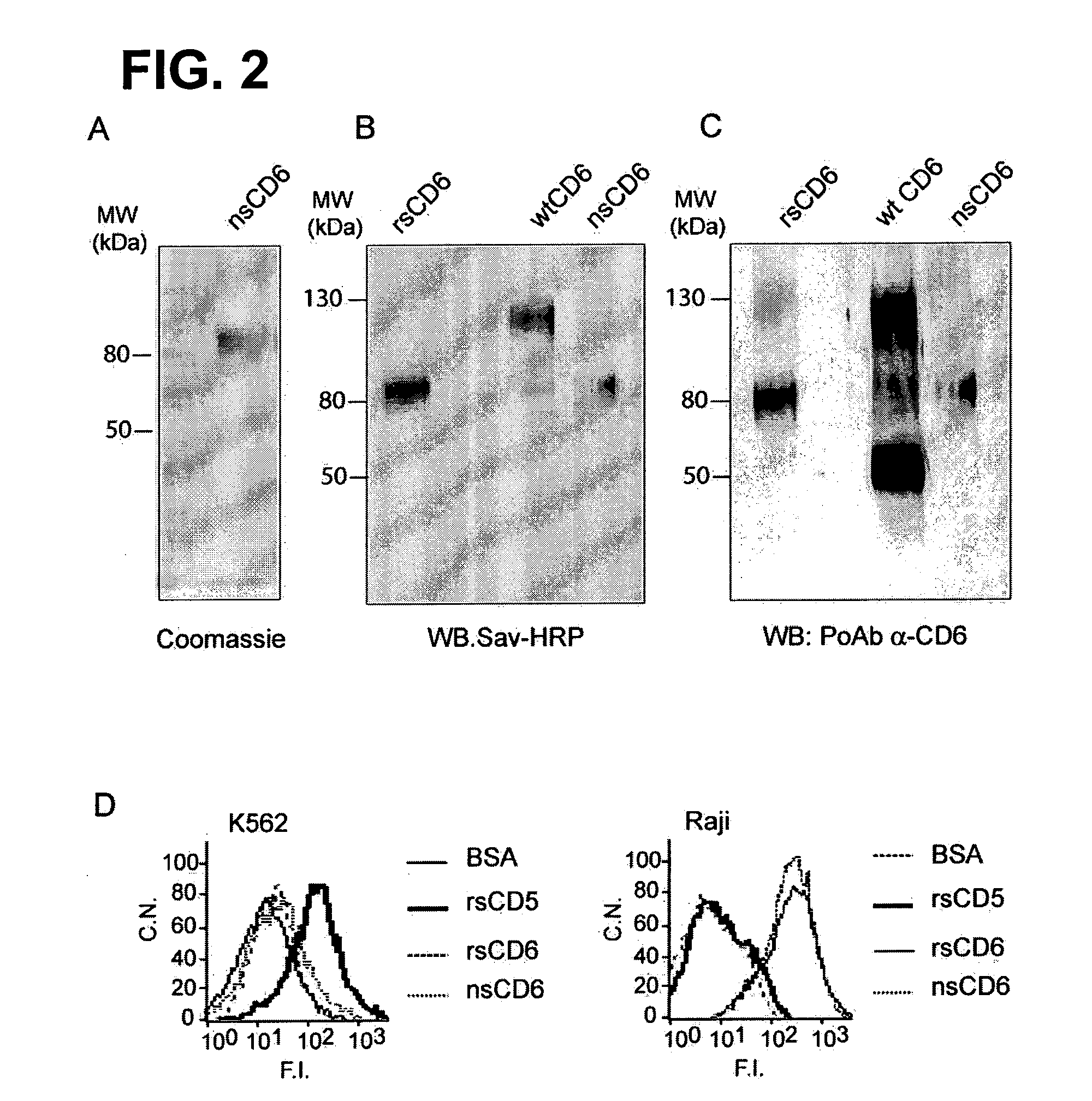
The inventors have found that CD6, a member of the Scavenger Receptor Cysteine-Rich (SRSR) superfamily expressed on human lymphocytes binds to Gram-positive and Gram-negative bacteria, as well as to other microbial structures. Thus, a CD6 product is useful for the manufacture of a medicament for therapeutic and / or preventive treatment of an infectious disease or of an inflammatory condition related to an infectious disease or to the presence of a product derived from an infectious agent in a mammal including a human. Examples of such inflammatory conditions are systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and septic shock.
Owner:SOTO FRANCISCO LOZANO DR +1
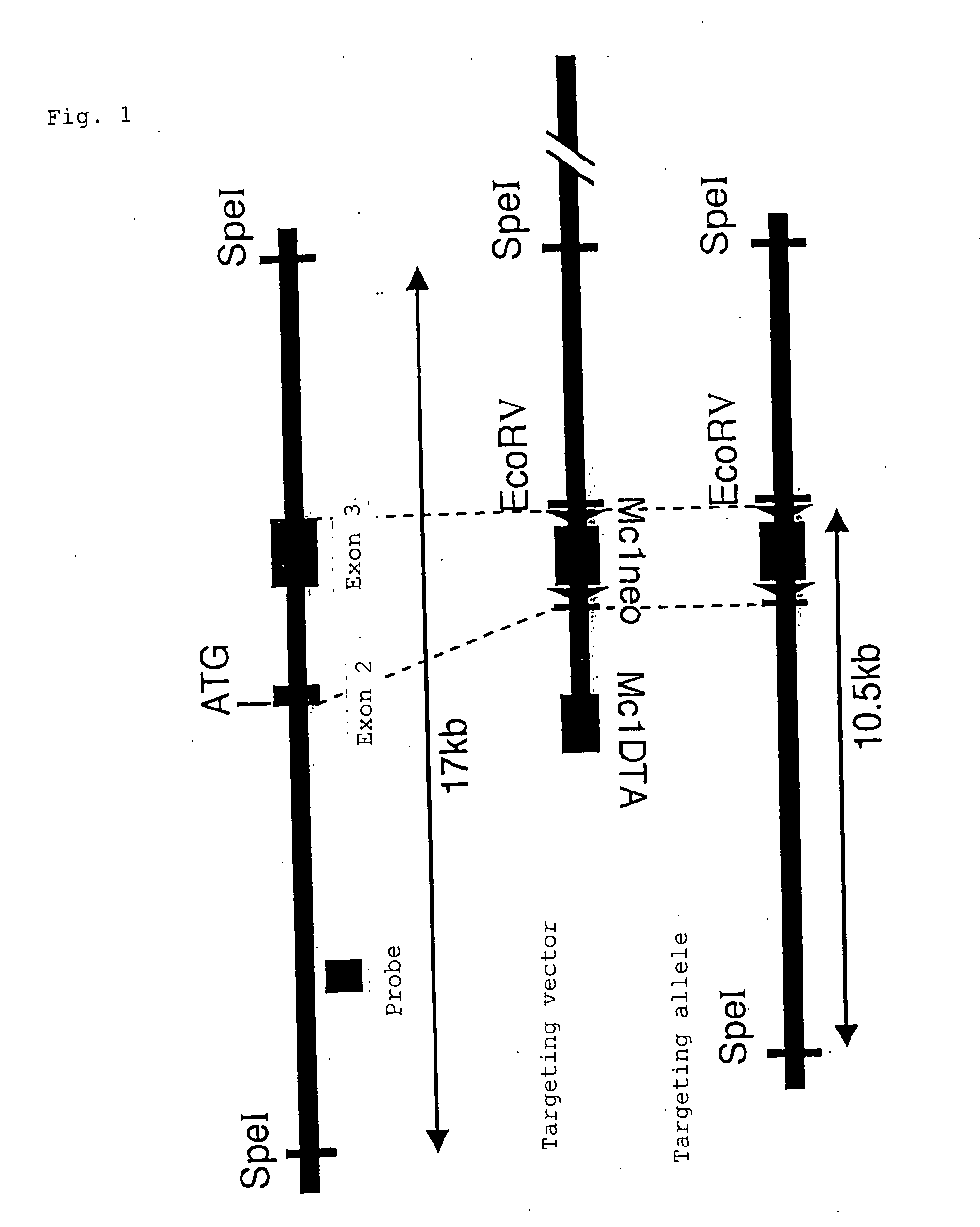
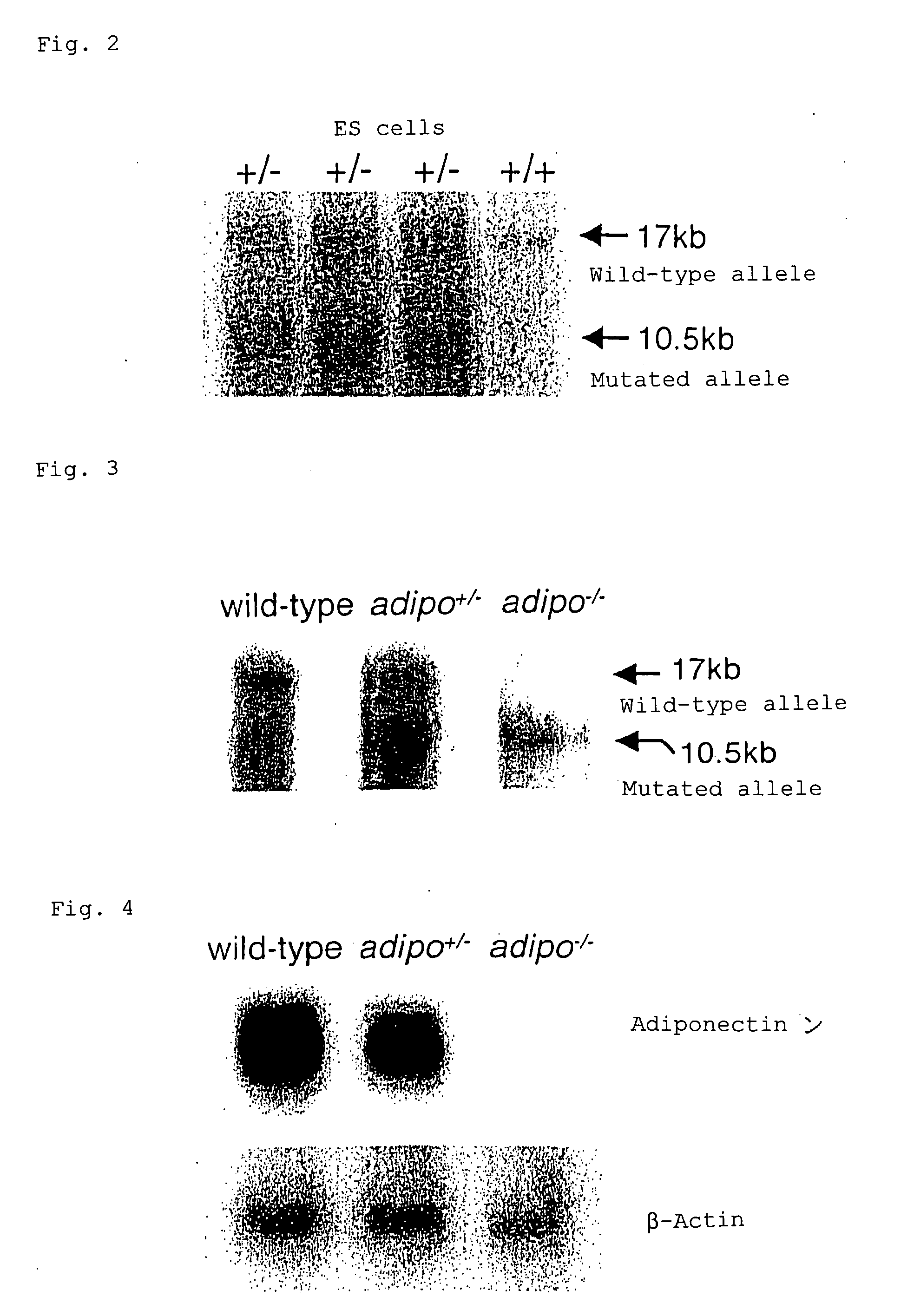
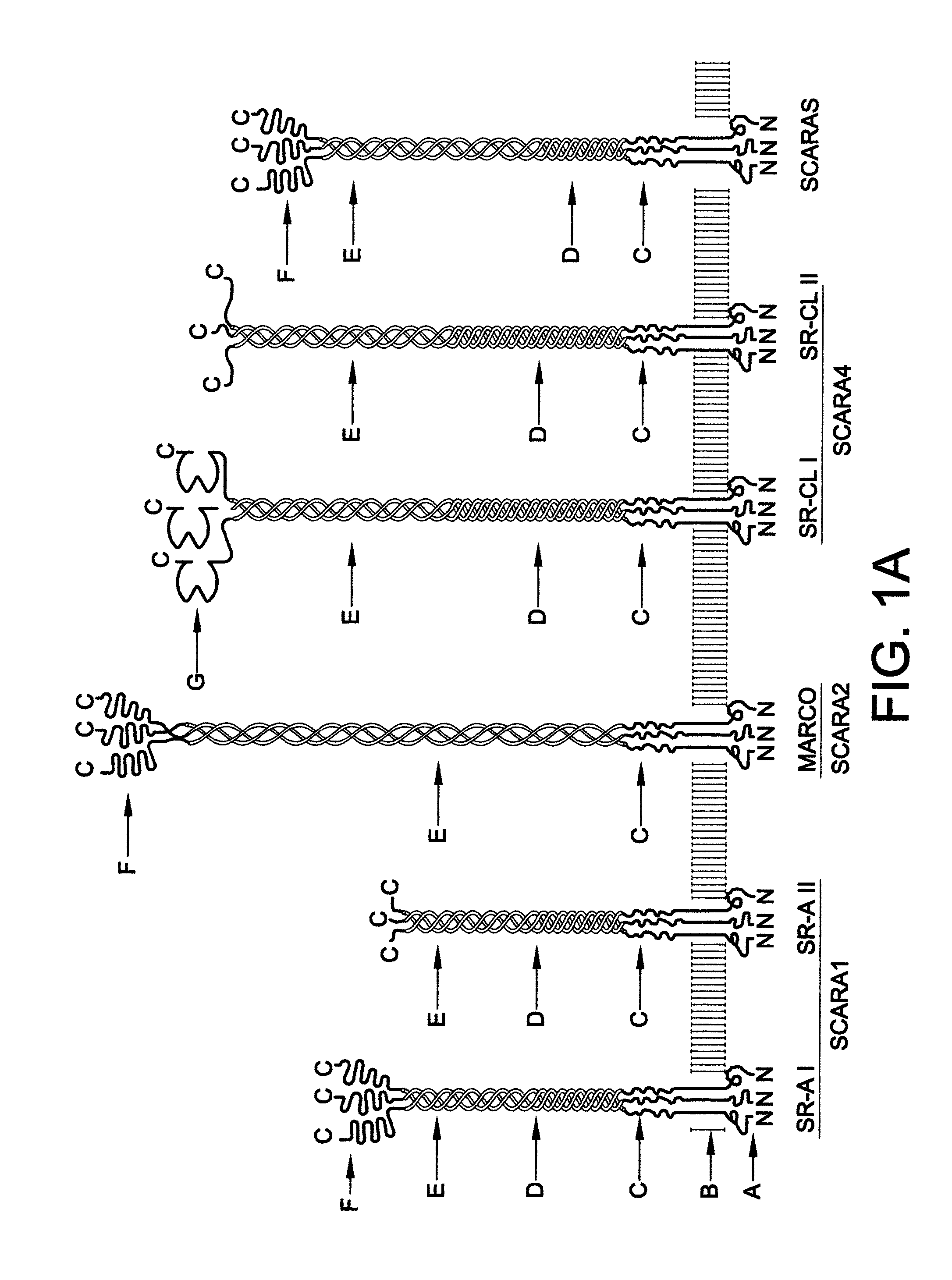
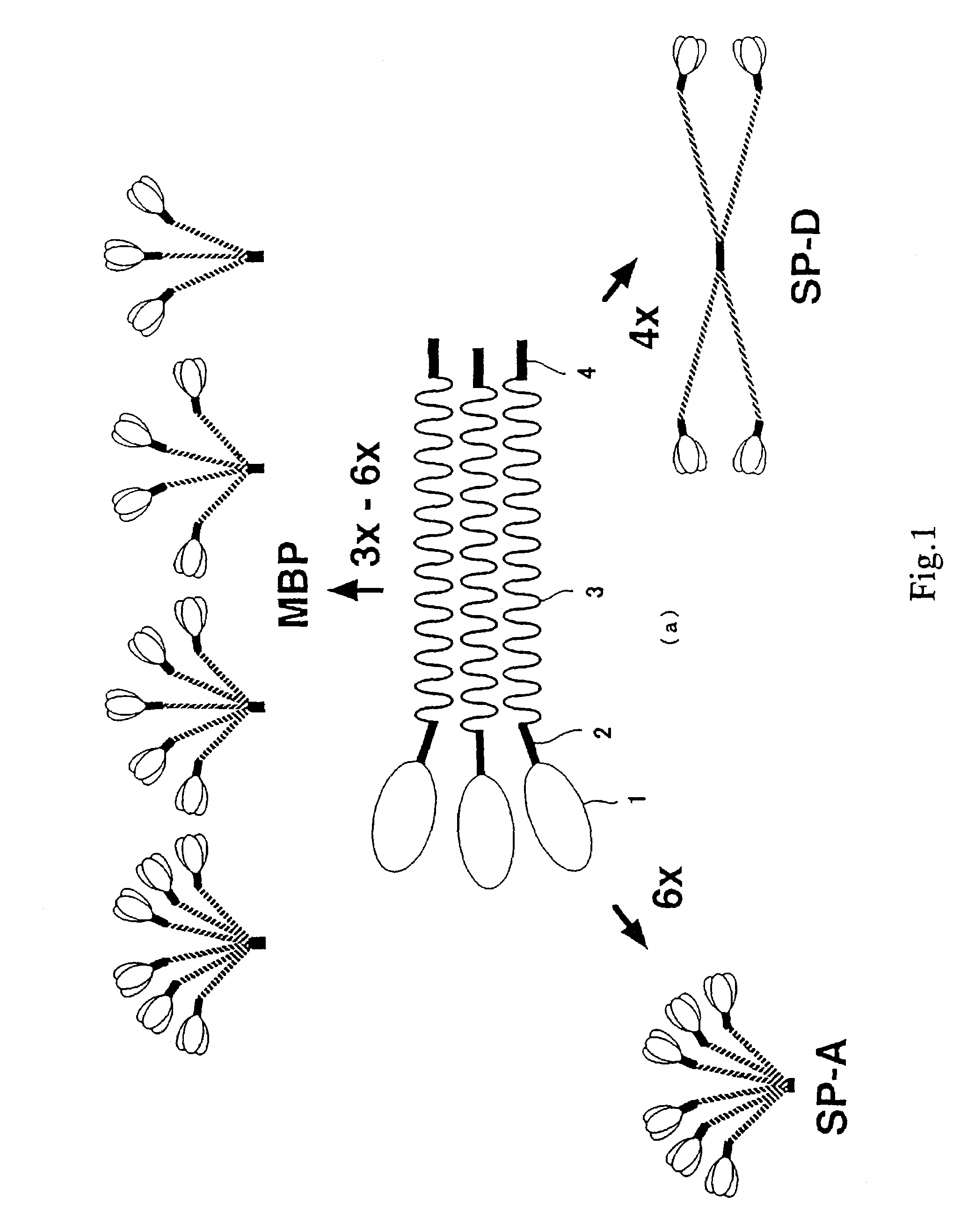
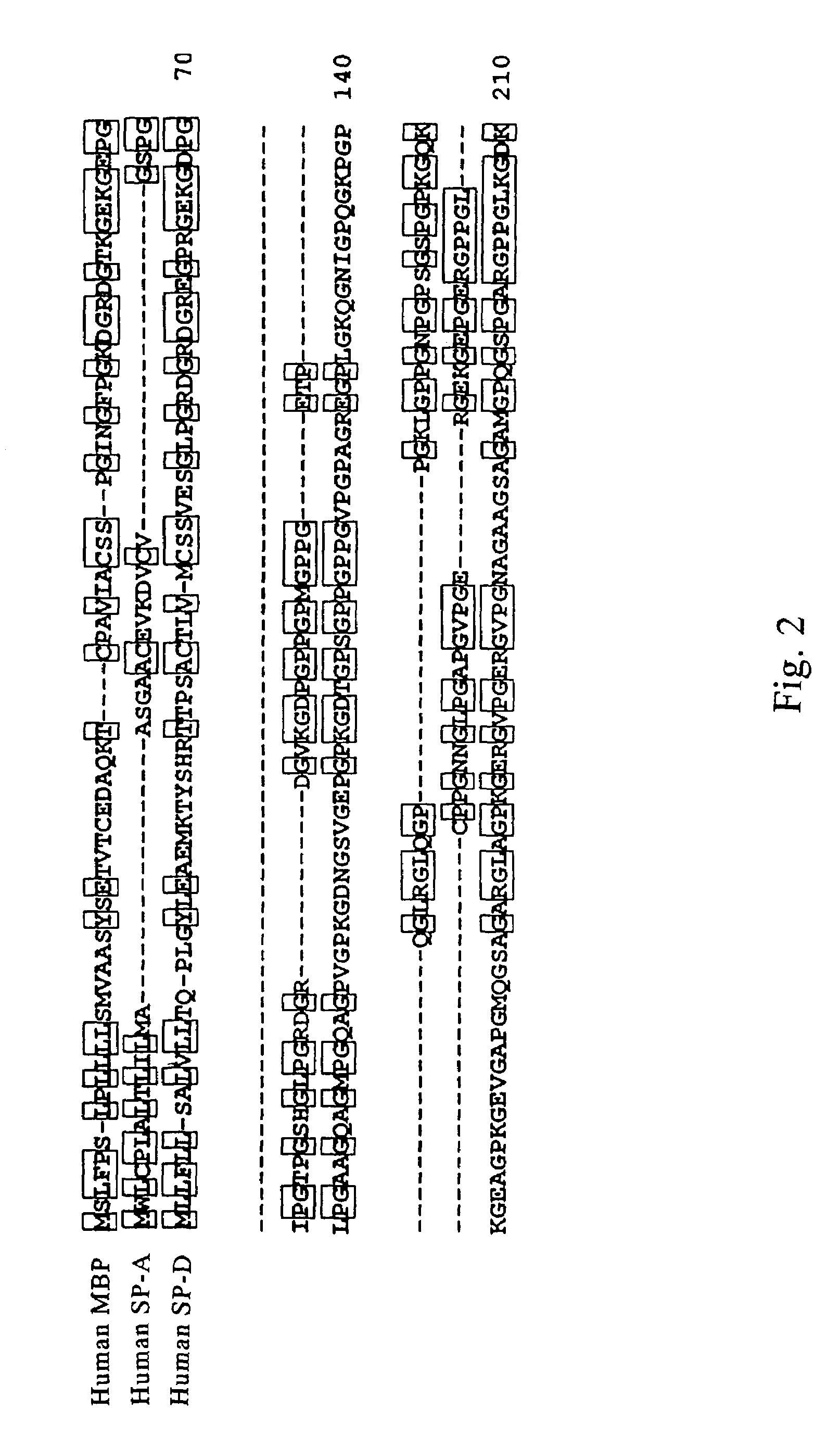
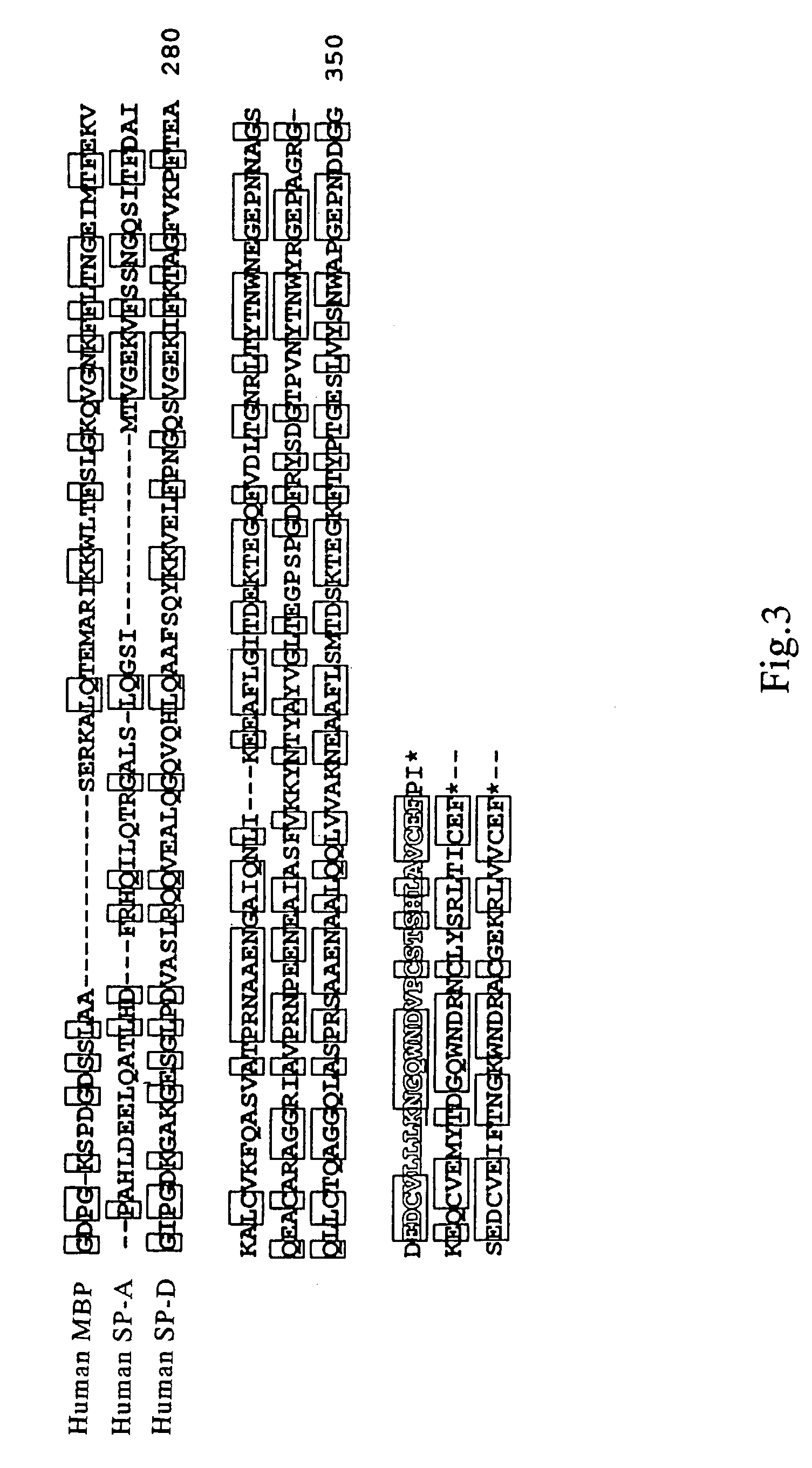
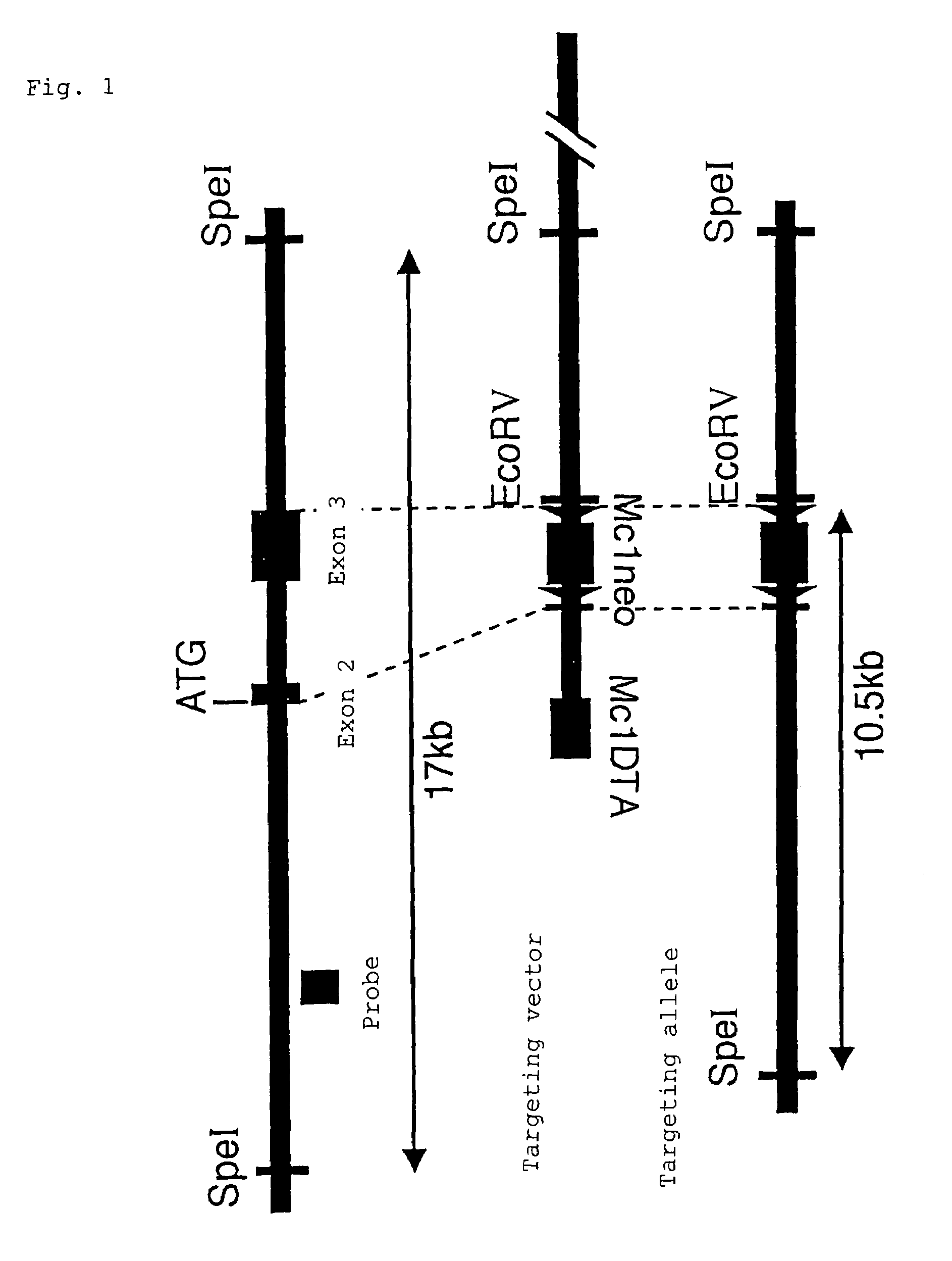
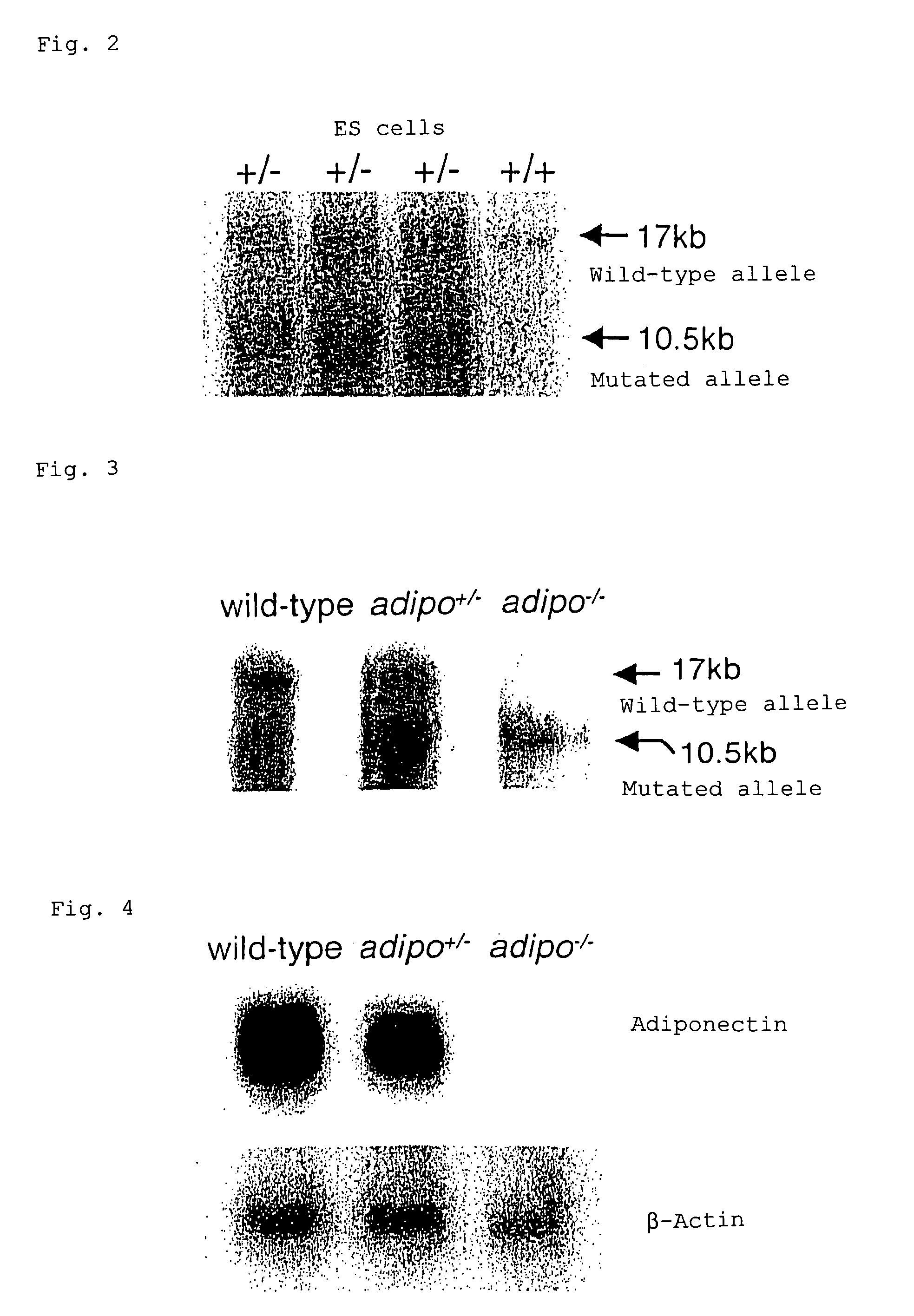
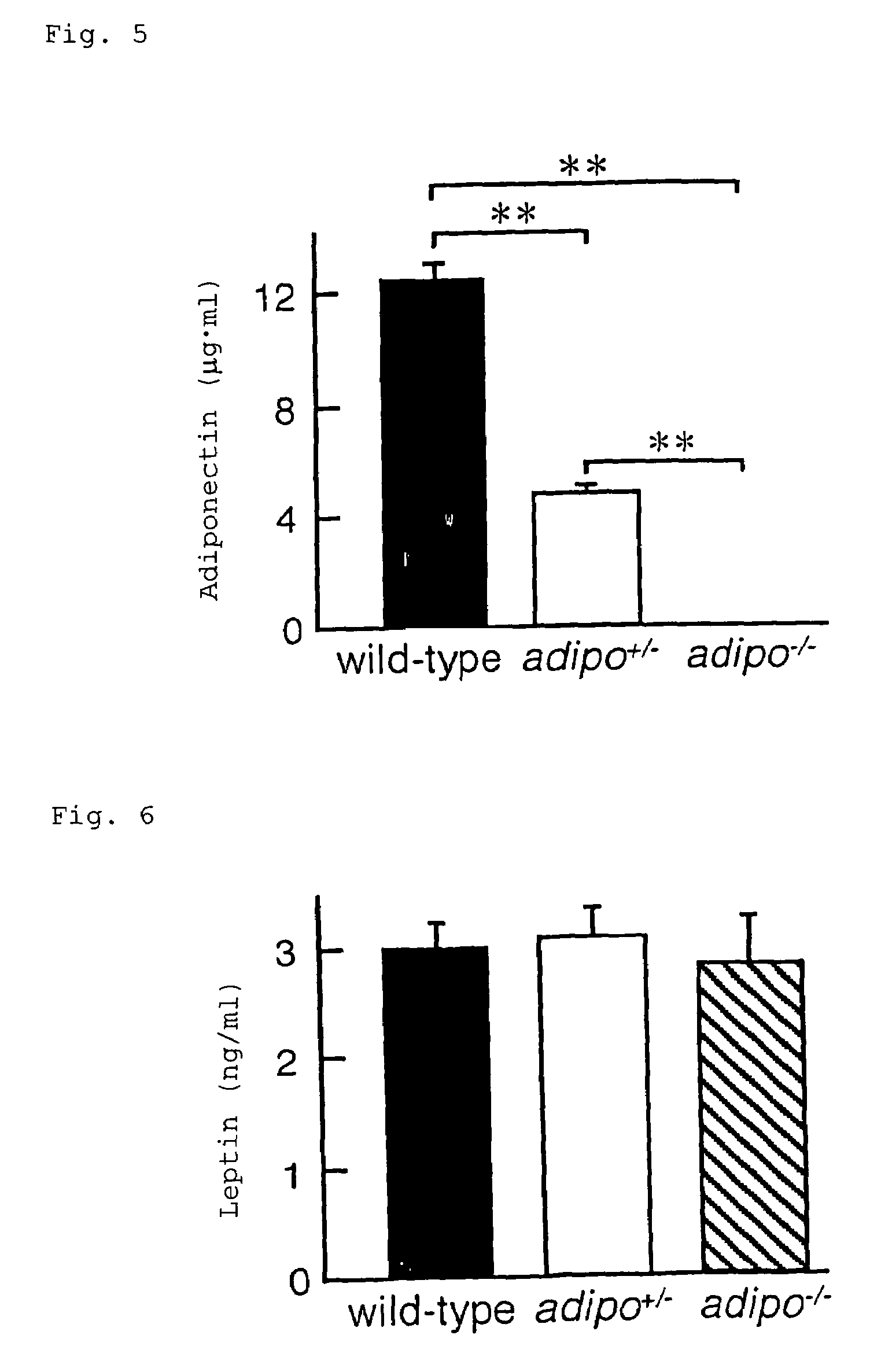
Scavenger receptors
Novel scavenger receptors having an SR structure and a collectin-like structure are provided, which can be utilized in the elucidation of mechanisms of macrophage and basic immunity; in the elucidation of mechanisms of the development of a wide variety of diseases such as arteriosclerosis, diabetic complications and Alzheimer's disease, hyper β-lipoproteinemia, hypercholesterolemia, hypertriglyceridemia, hypo α-lipoproteinemia, transplantation, atherectomy, post angiogenic restenosis, bacterial infections; in the diagnostic, prophylactic and therapeutic methods thereof; and in the development of reagents and drugs for the same. The novel scavenger receptors include proteins comprising an amino acid sequence set out in SEQ ID NO: 2, 4 or 24 or proteins having equivalent properties to the same, or derivatives or fragments thereof as well as isolated polynucleotides comprising a nucleotide sequence encoding these proteins, and related molecules such as antibodies, antagonists and the like. Also disclosed are methods for the treatment using the same.
Owner:FUSO PHARMA INDS
Method for treating arteriosclerosis
InactiveUS7419955B2Significantly thickened arterial intimaReduced expression levelPeptide/protein ingredientsMetabolism disorderScavenger receptorMedicine
A scavenger receptor A expression down-regulator and a drug for preventing or treating arteriosclerosis which contain, as the active ingredient, a C-terminal globular domain of adiponectin, adiponectin, or a gene encoding the domain or adiponectin. According to the present invention, there is provided a preventive or therapeutic agent capable of directly preventing intimal thickening, which constitutes an essential feature of arteriosclerosis. This effect can be attained through arresting the onset and development of arteriosclerosis by reducing the expression level of scavenger receptor A in arterial walls and preventing lipid buildup in macrophages.
Owner:JAPAN SCI & TECH CORP
Growth hormone-releasing peptides in the treatment or prevention of atherosclerosis and hypercholesterolemia
InactiveUS7785567B2Reducing the uptake of oxidised LDLReduce lesionsPeptide/protein ingredientsMetabolism disorderScavenger receptorCellular cholesterol
According to the invention there is provided a method of treatment or prophylaxis of atherosclerosis, hypercholesterolemia or a cardiovascular disease associated with atherosclerosis, which method comprises administration of one or more Growth Hormone Releasing Peptides (GHRPs) to a patient in need of such treatment or prophylaxis. There are also provided methods of reducing blood plasma cholesterol levels, as well as methods of modulating the expression of the scavenger receptor CD36 and genes involved in cellular cholesterol efflux.
Owner:VALORISATION HSJ SOC & COMMANDITE CO CHU SAINTE JUSTINE LE CENT HOSPITALIER UNIV MERE ENFANT
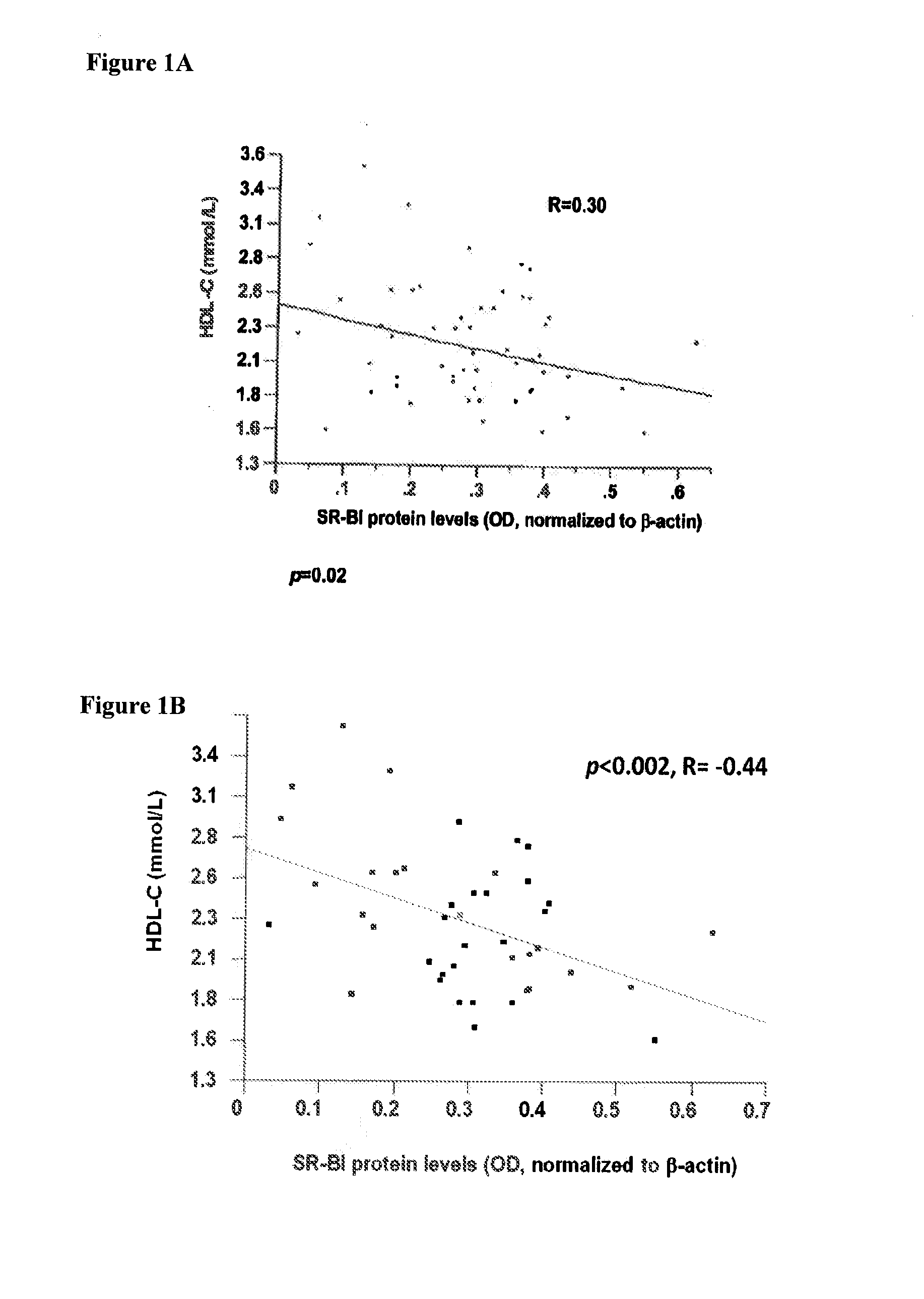
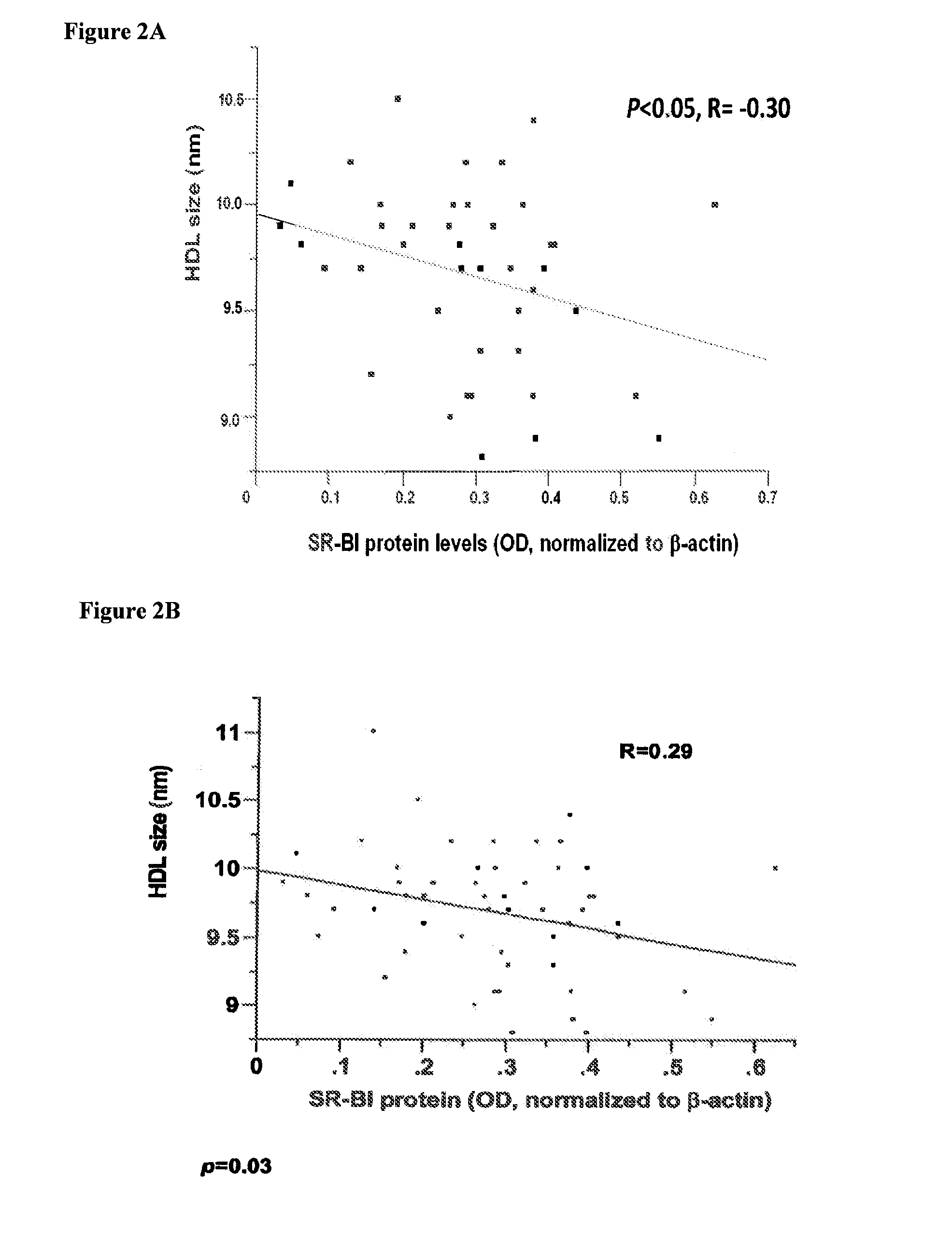
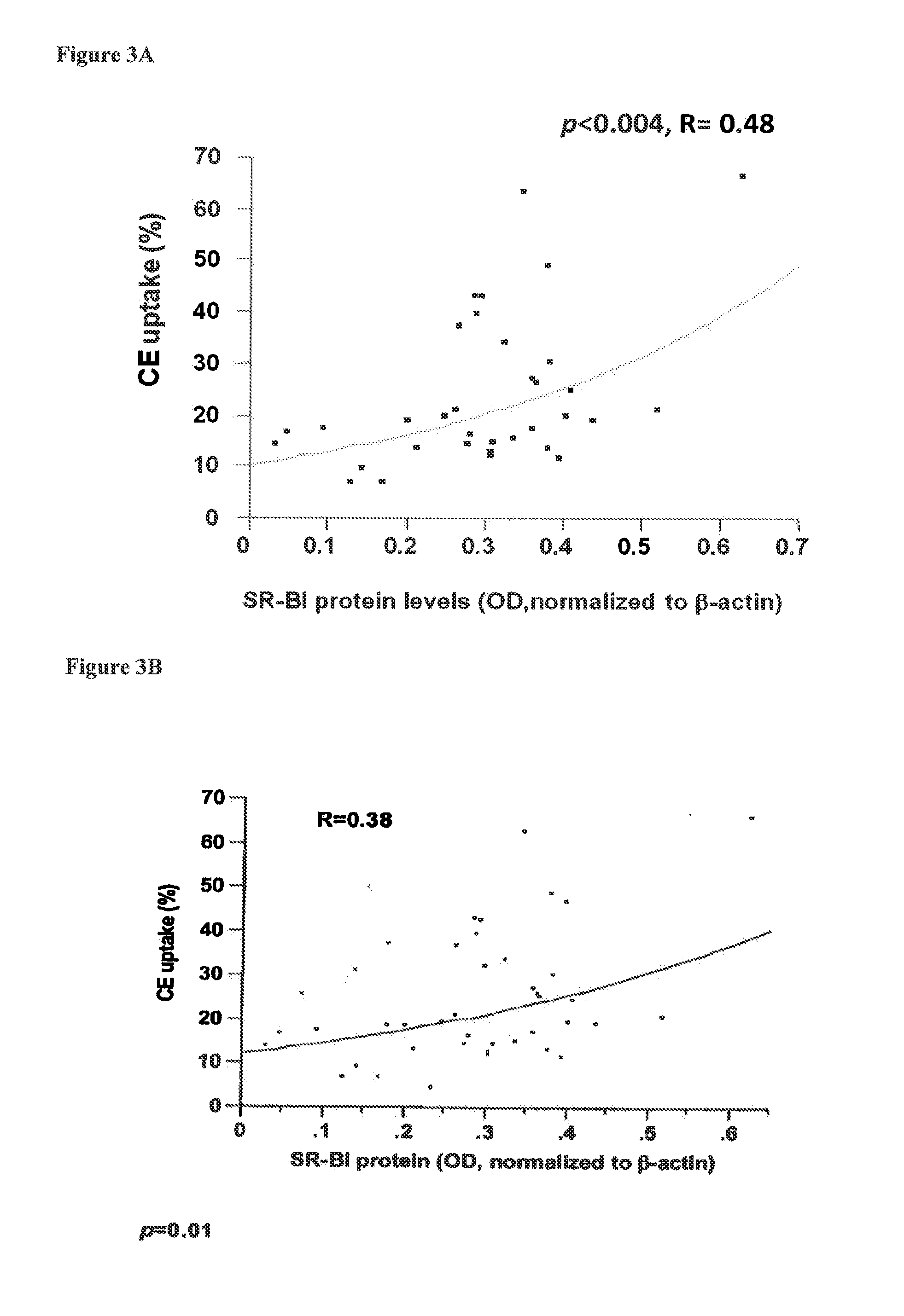
Sr-bi as a predictor of elevated high density lipoprotein and cardiovascular disease
The present invention relates to the field of single nucleotide polymorphisms (SNPs) and uses therefore as a predictor of diseases and conditions. Specifically, the present invention provides methods and kits useful in determining whether a subject is at increased risk for developing a cardiovascular disease by screening for the presence of a SNP in the scavenger receptor class B type I (SR-BI) gene of a subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Nanostructures for treating cancers and other conditions
Nanostructures, compositions and methods for treating cancers and other conditions are provided. In some cases, the nanostructures and / or compositions may be used to treat cancers or other diseases or conditions at least in part by controlling cholesterol metabolism in cells. The nanostructures and compositions may be used, for example, to control cholesterol influx and / or efflux in cells. In some cases, the nanostructures or compositions may be used to kill the cells. Examples of cancer cells that may be affected by the nanostructures and compositions described herein include those having scavenger receptor type B-I (SR-B1), B-cell lymphoma cells, non-Hodgkin's lymphoma cells, melanoma cells and / or others. In some embodiments, the nanostructures may be mimics of natural high-density lipoprotein (HDL).
Owner:NORTHWESTERN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com