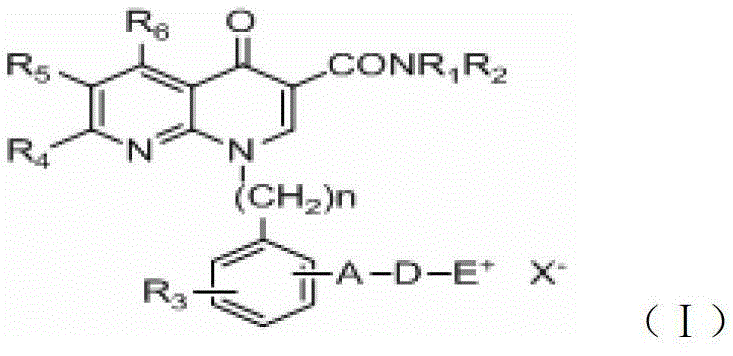
A group of 1-substituted-1, 8-naphthyridine formamide derivatives and preparation and application thereof
A kind of technology of naphthyridine carboxamide and derivatives, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
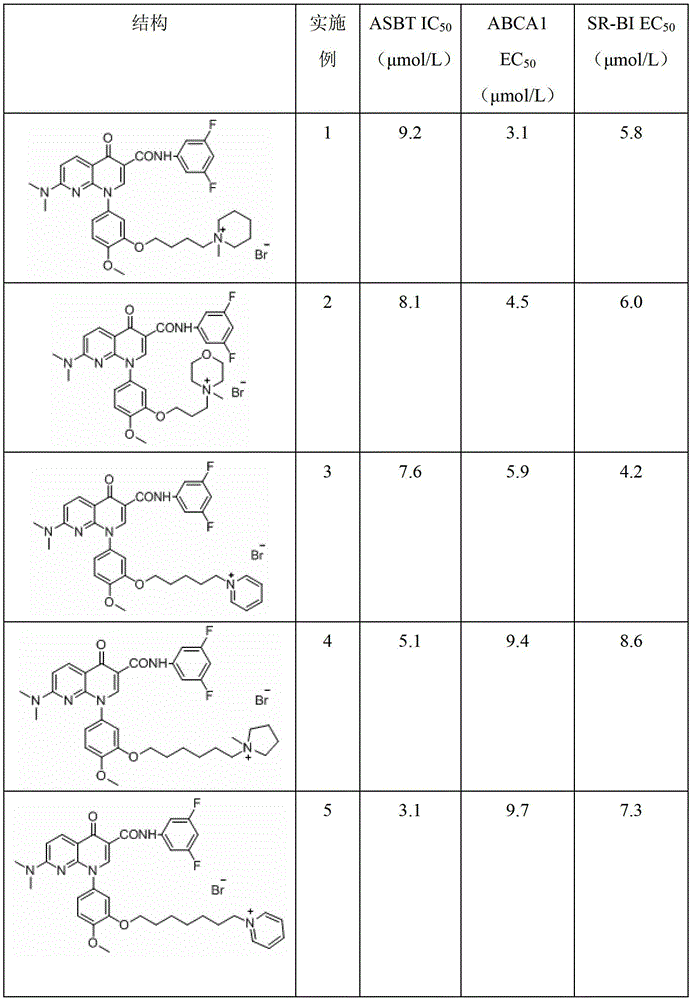
Examples
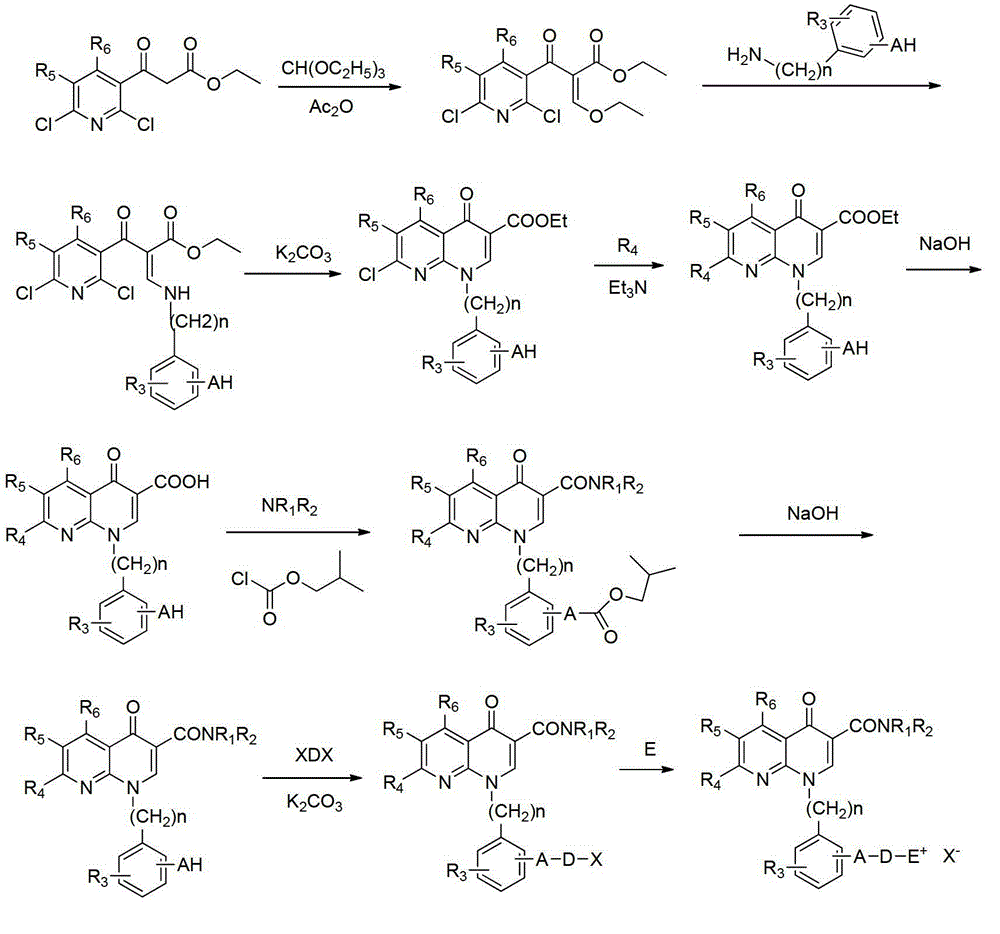
Embodiment 1
[0036] (a) Synthesis of 2-(2,6-dichloro-nicotinoyl)-3-(3-hydroxy-4-methoxyanilino)-ethyl acrylate
[0037] Add ethyl 2,6-dichloronicotinoyl acetate (10g, 38.2mmol) to 20mL of acetic anhydride, then add triethyl orthoformate (11.2g, 76.3mmol), heat up to 120°C and stir for 2 hours, The reaction solution was evaporated under reduced pressure to remove the solution, then cooled to room temperature, 20 mL of dichloromethane was added, after stirring evenly, 3-hydroxy-4-methoxyaniline (3.7 g, 26.7 mmol) was added, and the reaction was performed for 2 hours. Wash the filter cake with a small amount of ether, and dry the filter cake in vacuum to obtain 6.3 g of yellow powder solid, with a yield of 40.1%.
[0038] 1 H-NMR(DMSO)δ:1.01(t,J=7.2Hz,3H),3.79(s,3H),3.98-4.01(m,2H),6.04(s,1H),6.60(d,J=7.2 Hz,1H),6.91(d,J=7.2Hz,1H),7.73(d,J=7.4Hz,1H),8.17(d,J=7.4Hz,1H),8.63(d,J=13.8Hz, 1H),12.35(d,J=13.8Hz,1H).MS m / z:412.17[M+H] + .
[0039](b) Synthesis of ethyl 1-(3-hydroxy-4-methoxyphe...
Embodiment 2
[0061] (a) 1-[3-(3-bromopropoxy)-4-methoxyphenyl]-7-(dimethylamino)-1,4-dihydro-4-oxo-1,8 Synthesis of -naphthyridine-3-(3,5-difluorophenyl)carboxamide
[0062] Prepared according to the method of step (g) of Example 1, using the product of step (f) of Example 1 and 1,3-dibromopropane to obtain 1-[3-(3-bromopropoxy)-4-methane Oxyphenyl]-7-(dimethylamino)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-(3,5-difluorophenyl)carboxamide compound, It is in the form of white powder with a yield of 76%.
[0063] 1 H-NMR (CDCl 3 ):1.97-1.98(m,2H),3.09(s,6H),3.56-3.57(m,2H),3.80(s,3H),4.07-4.08(m,2H),6.52(t,J=1.2 Hz,1H),6.65(d,J=5.6Hz,1H),7.09(d,J=6.0Hz,1H),7.23(dd,J 1=6.0Hz,J 2 =1.6Hz,1H),7.35(d,J=1.6Hz,1H),7.37(d,J=1.2Hz,1H),7.39(d,J=1.6Hz,1H),8.41(d,J=6.0 Hz,1H),8.70(s,1H).MS m / z:588.32[M+H] + .
[0064] (b) 1-{3-[3-(N-methylmorpholine bromide)]propoxy-4-methoxyphenyl}-7-(dimethylamino)-1,4-dihydro Synthesis of -4-oxo-1,8-naphthyridine-3-(3,5-difluorophenyl)carboxamide
[0065] Pre...
Embodiment 3
[0068] (a) 1-[3-(5-Bromopentyloxy)-4-methoxyphenyl]-7-(dimethylamino)-1,4-dihydro-4-oxo-1,8 Synthesis of -naphthyridine-3-(3,5-difluorophenyl)carboxamide
[0069] Prepared according to the method of step (g) of Example 1, using the product of step (f) of Example 1 and 1,5-dibromopentane to obtain 1-[3-(5-bromopentyloxy)-4- Methoxyphenyl]-7-(dimethylamino)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-(3,5-difluorophenyl)carboxamide compound , in the form of off-white powder, with a yield of 77%.
[0070] 1 H-NMR (CDCl 3 ):1.29-1.30(m,2H),1.78-1.80(m,4H),3.08(s,6H),3.50-3.51(m,2H),3.80(s,3H),4.06-4.07(m,2H ),6.51(t,J=1.2Hz,1H),6.65(d,J=5.6Hz,1H),7.08(d,J=6.0Hz,1H),7.26(dd,J 1 =6.0Hz,J 2 =1.6Hz,1H),7.35(d,J=1.6Hz,1H),7.36(d,J=1.2Hz,1H),7.39(d,J=1.6Hz,1H),8.46(d,J=6.0 Hz,1H),8.71(s,1H).MS m / z:616.36[M+H] + .
[0071] (b) 1-{3-[5-(Pyridine bromide)pentyloxy]-4-methoxyphenyl}-7-(dimethylamino)-1,4-dihydro-4-oxo Synthesis of -1,8-naphthyridine-3-(3,5-difluorophenyl)carboxamide
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com