Preparation and application of gene and chemotherapeutic drug co-delivery anti-tumor nanoparticle of target SR-BI (Scavenger Receptor-BI)
A chemotherapeutic drug and nanoparticle technology, applied in gene therapy, antineoplastic drugs, drug combinations, etc., can solve the problems of difficult extraction, easy contamination of blood products and biological safety, and achieve the effect of improving encapsulation efficiency and efficient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of rHDL drug-loaded nanoparticles
[0030]
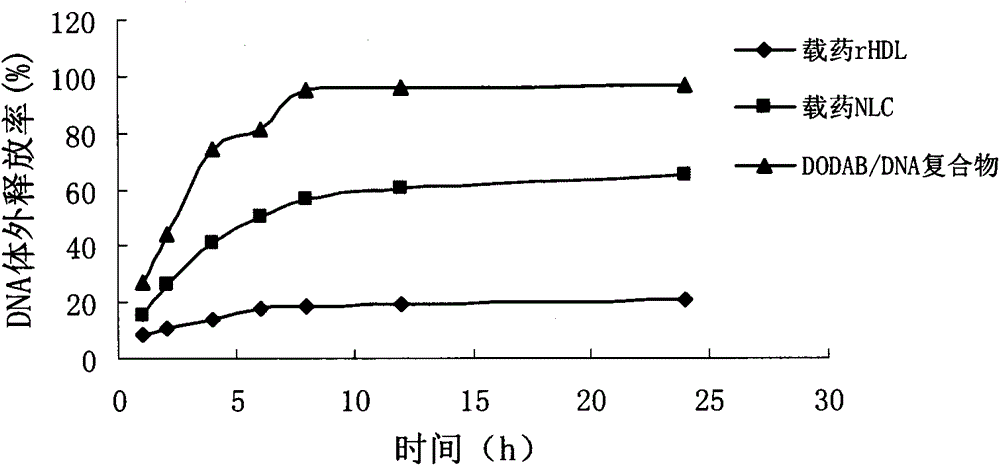
[0031] Accurately measure the plasmid DNA to make a 0.5mg / ml solution, and dissolve DODAB in ethanol to make a 3.88mg / ml ethanol solution. According to the ratio of N / P ratio of 4, the DNA solution was injected into the DODAB ethanol solution to obtain the DODAB / DNA complex, vortexed for 30 s, and stood at room temperature for 30 min.
[0032] N / P=(m DODAB × M DNA ) / (m DNA × M DODAB )
[0033] where m DODAB is the mass of DODAB, m DNA is the mass of DNA, M DODAB is the relative molecular mass of DODAB, M DNA is the relative molecular mass of DNA.
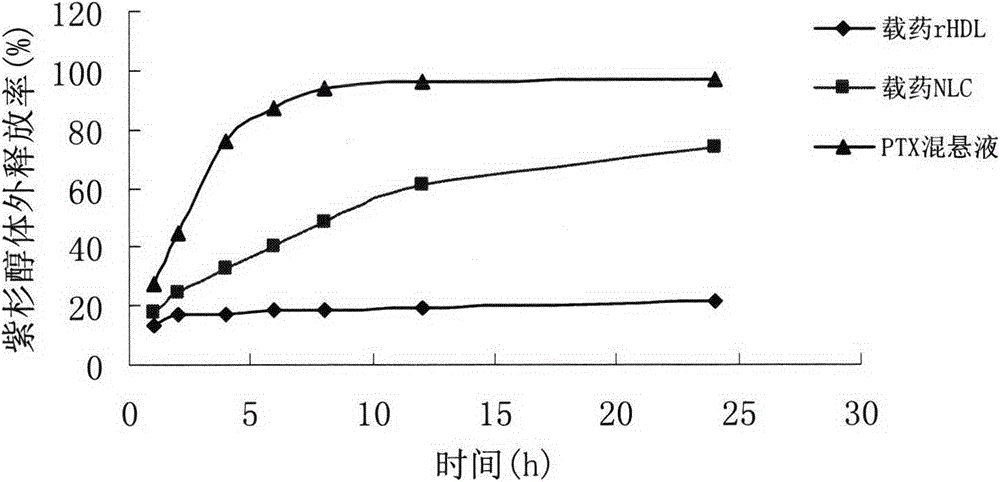
[0034] Dissolve the prescribed amount of phospholipids, cholesterol, and cholesteryl ester in chloroform, add paclitaxel methanol solution, and dry the organic solvent by rotary evaporation at 37°C, and then put it in a vacuum desiccator overnight to remove the residual solvent. Add pH 7.4 PBS buffer containing DODAB / DNA, hydrate until a suspension...
Embodiment 2
[0035] Example 2: Research on the properties of rHDL drug-loaded nanoparticles
[0036] 2.1 Particle size and morphology of rHDL drug-loaded nanoparticles
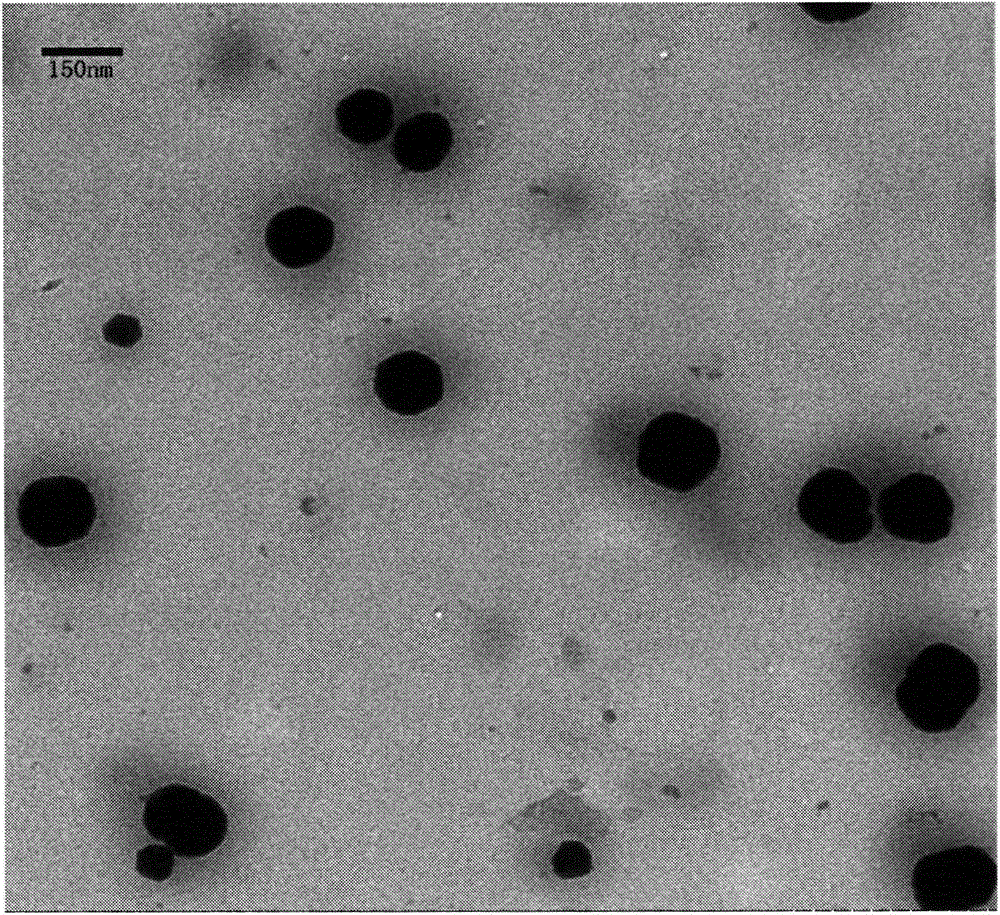
[0037] The average particle size of the complex was measured by a laser particle size analyzer to be 184 nm. Transmission electron microscope results are as figure 1 As shown, the prepared rHDL drug-loaded nanoparticles are all spherical particles with good roundness and smooth appearance. The measured particle size is about 150nm, which is smaller than that measured by the laser particle size analyzer. The reason may be that the surface band There is an electric double layer around the nanoparticles with a certain negative charge, and the electric double layer can scatter light, resulting in larger results measured by the laser particle size analyzer. The protein on the surface of rHDL drug-loaded nanoparticles contains a large number of hydrophilic amino groups, which will bind more water molecules to form a thicker hy...
Embodiment 3
[0047] Example 3: Cytotoxicity Test (MTT)
[0048] 3.1 Cytotoxicity test of the carrier
[0049] MCF-7 cells in good growth state and in the logarithmic growth phase were taken, digested with 0.25% trypsin, and blown into a single cell suspension. Adjust the cell concentration to 1 x 10 5 1 / ml, 100 μl per well was inoculated in a 96-well plate, and 5 duplicate wells were set up. Place the cell plate at 37°C, 5% CO 2 cultured overnight in a cell culture incubator. Discard the medium, add 100 μl of fresh medium, then add 100 μl of blank NLC, blank rHDL, DODAB solution diluted in serum-free medium, continue to cultivate for 24 hours, suck off the medium, and add 20 μl of 0.5 mg / ml MTT solution to each well , 37°C, 5% CO 2 Incubate for 4 h in a cell culture incubator. The supernatant was discarded, and 150 μl of dimethyl sulfoxide (DMSO) solution was added to each well, and shaken for 10 min. The absorbance value of each well was measured at 570 nm with an enzyme-linked imm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com