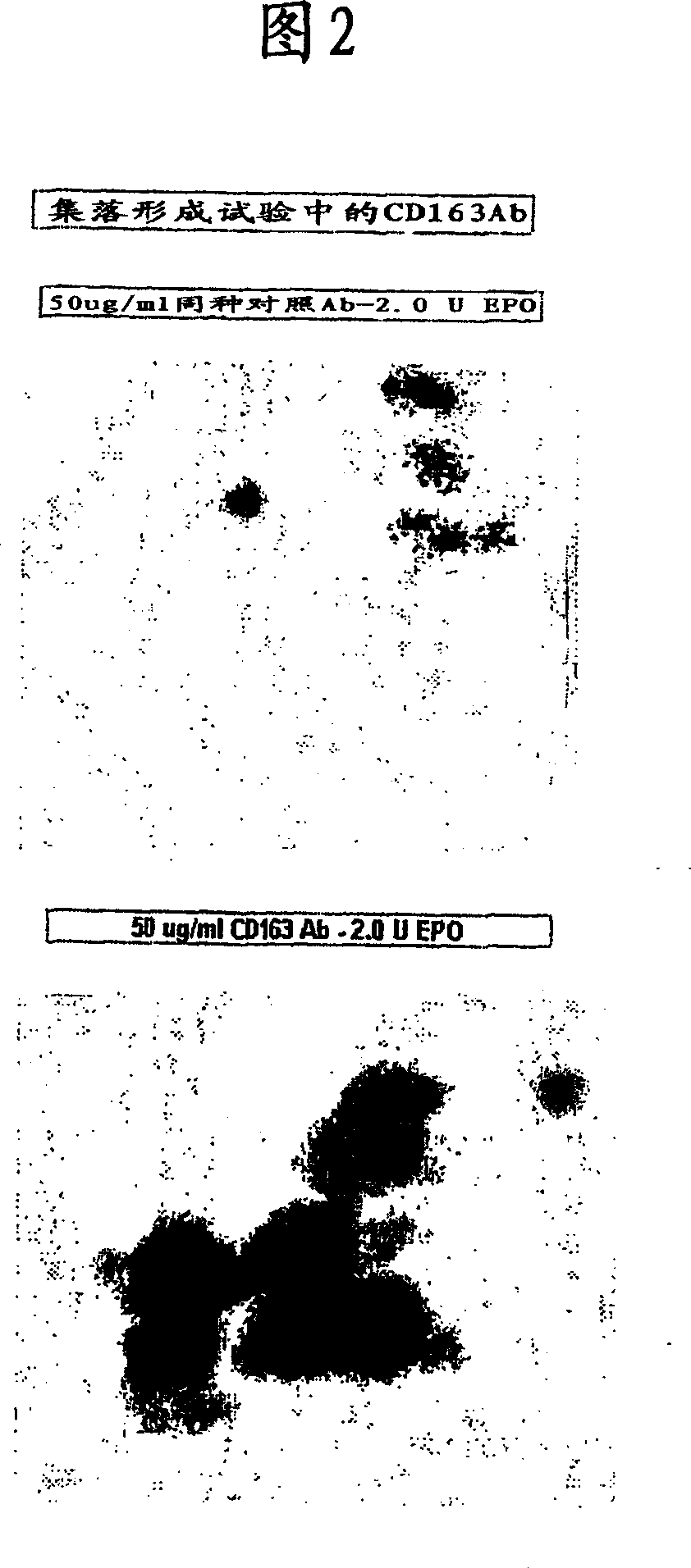
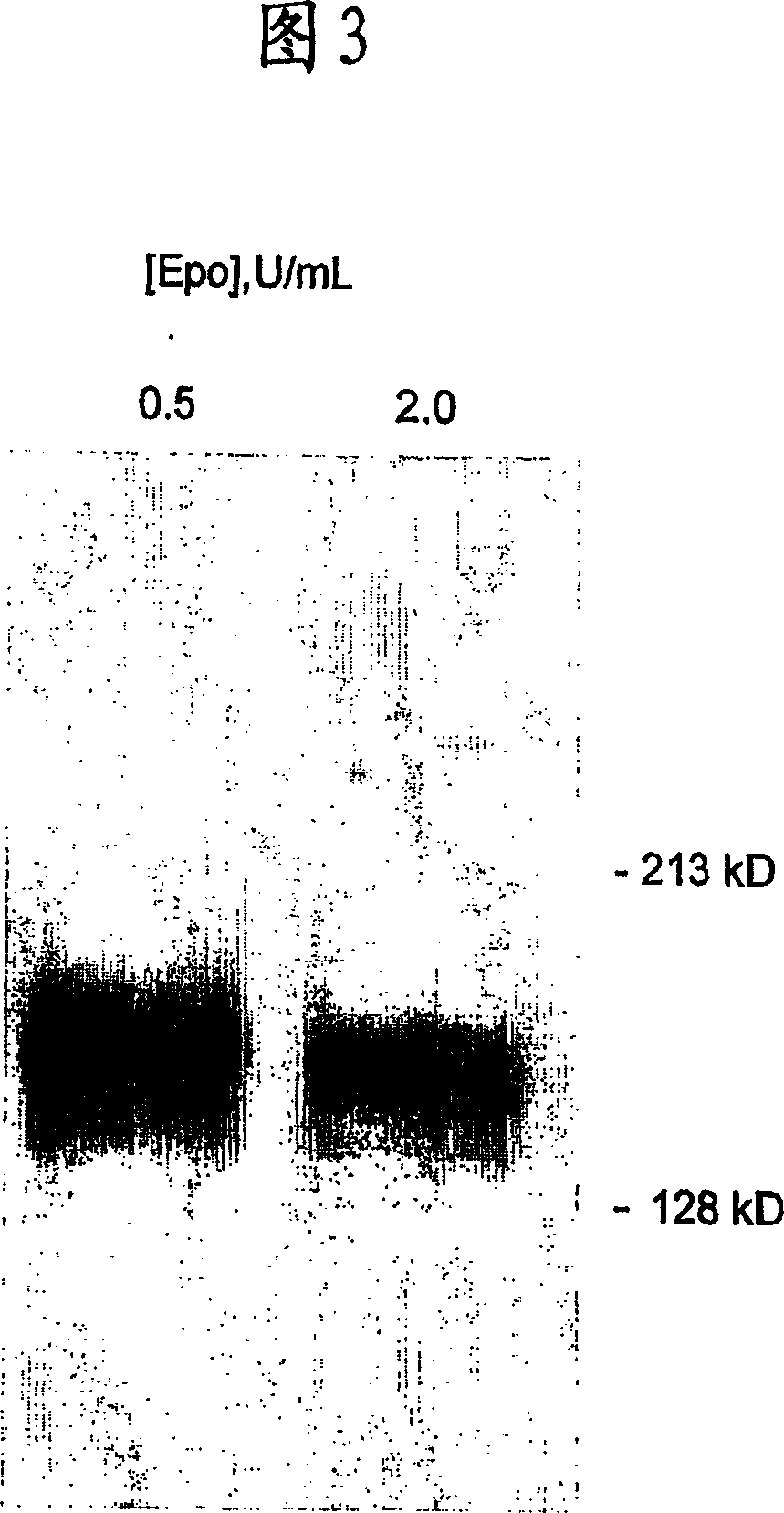
Blood cell production via activation of the hemoglobin scavenger receptor
A technology of erythrocyte lineage and cells, applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., can solve the problem of no response to Epo treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The pharmaceutical compositions may be prepared by methods known per se for the preparation of pharmaceutically acceptable compositions which can be administered to patients, thus combining an effective amount of the active substance in admixture with pharmaceutically acceptable excipients. For example, Remington's Pharmaceutical Sciences describes suitable excipients (Remington's Pharmaceutical Sciences, Mack Publishing Company, Easton, Pa., USA 1985).
[0077] On this basis, the pharmaceutical composition includes, but is not limited to, the active compound or substance in combination with one or more pharmaceutically acceptable excipients or diluents, and contained at a suitable pH and isotonic with physiological fluids. in the buffer. The pharmaceutical composition also additionally comprises other agents, eg other agents that can stimulate hematopoiesis, erythropoiesis or myelopoiesis, or that can stimulate the growth, proliferation, differentiation and / or mobiliza...
Embodiment 1
[0088] CD34 + Cells express CD163
[0089] CD34 from cryopreserved adult bone marrow (ABM) + Cells were permeabilized with Cytofix / Cytoperm solution (Pharmingen Transduction Laboratories, adivision of BD BioSciences; Becton, Dickson and Company) at 4°C for 30 minutes, washed with fluorescently labeled isotype control antibody (IgG1-FITC) or fluorescently labeled Mac-158 Mouse anti-human CD163 monoclonal antibody (MBS; Mac158-FITC) incubation. In parallel experiments, cells from the same bone marrow samples were also stained extracellularly with fluorescently labeled anti-CD34 (HPCA-2-PE) and anti-CD45 (H130-FITC). Samples were analyzed using an EPICSXL flow cytometer (Beckman Coulter, Inc). The results are shown in Table 1, indicating that more than 95% of myeloid cells are CD34 / CD45 + , most of these cells can also be stained with anti-CD163 antibody.
[0090] Table 1: CD34 + Flow Cytometry Analysis of Bone Marrow Cells
[0091] % CD45 + / CD34 +
Embodiment 2
[0093] Western blot detects CD34 + CD163 expressed by cells
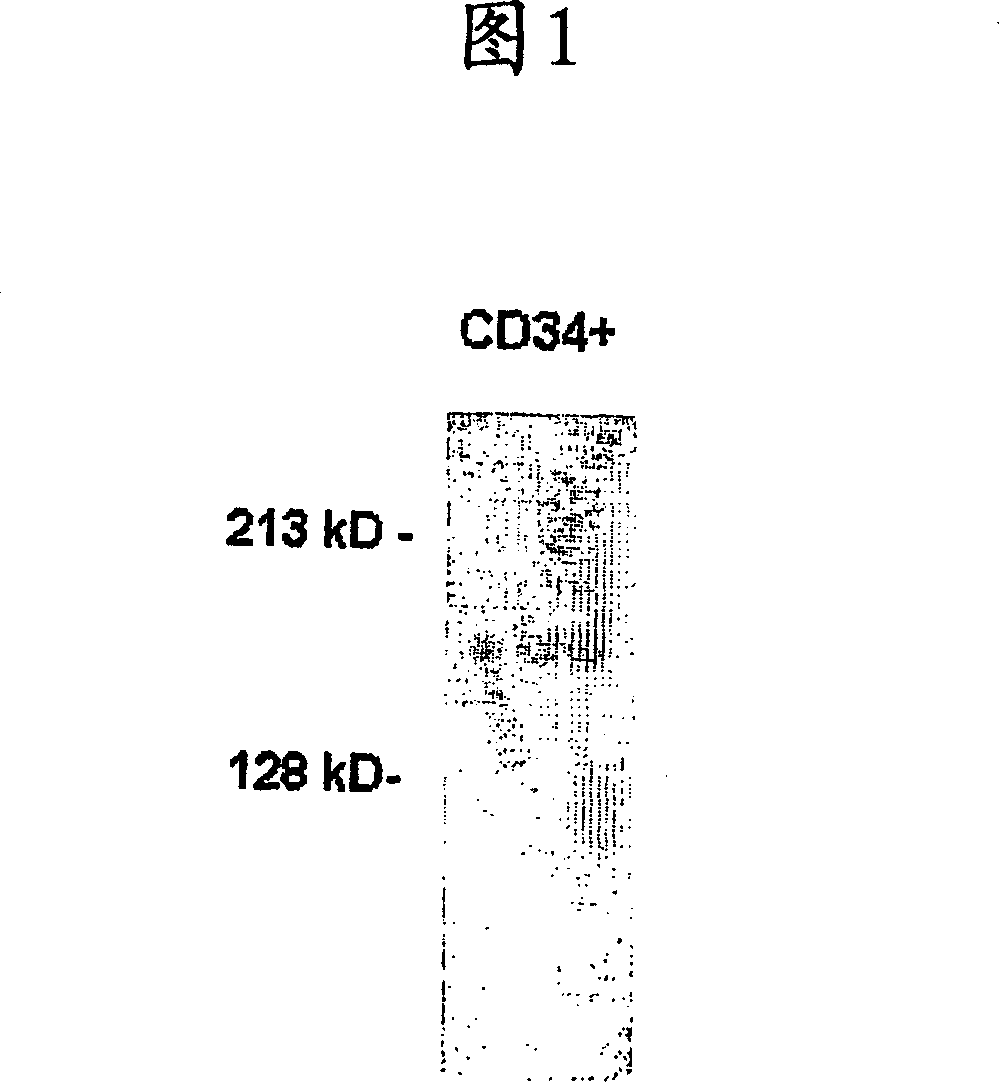
[0094] Resuspend adult bone marrow-derived CD34 in CHAPS buffer containing protease inhibitor cocktail (0.5% CHAPS, 10 mM Tris, 1 mM MgC12, 1 mM EDTA, and 10% glycerol) + cells to prepare cell lysates. Resuspend cells on ice for 30 minutes. Centrifuge the lysate and transfer the supernatant to a new tube. Resuspend the supernatant in non-reducing SDS loading buffer for Western blot analysis. Samples were then run through an 8% polyacrylamide gel and transferred to a nylon membrane. Nylon membranes were blocked with a solution containing nonfat dry milk and then detected with Mac-158 anti-human CD163 antibody followed by goat anti-mouse antibody conjugated to horseradish peroxidase (BIO-RAD Laboratories, Inc.). Bound secondary antibodies were detected using an enhanced chemiluminescence kit from Amersham plc.
[0095] As shown in Figure 1, from CD34 + CD163 immunoreactivity was detected in cell lysates prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com