Macrocyclic compounds as HCV entry inhibitors
A technology of compounds and inhibitors, applied in the field of new compounds of formula I, can solve the problem that the degree of correlation between viruses and cellular factors is not fully understood, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
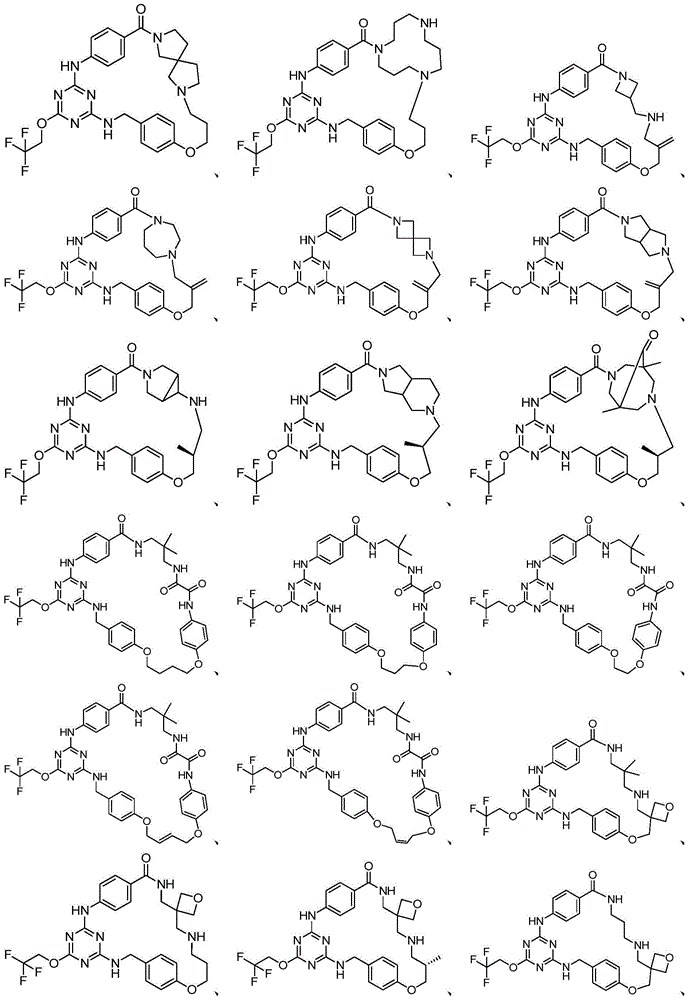
Examples
Embodiment Construction
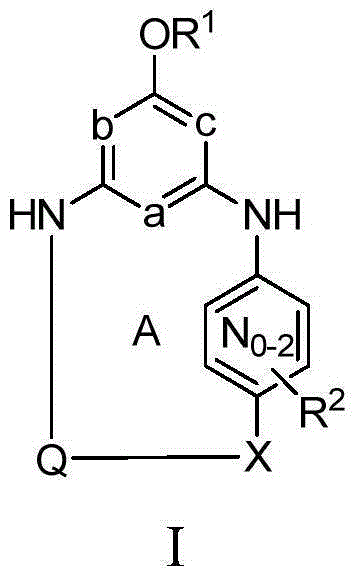
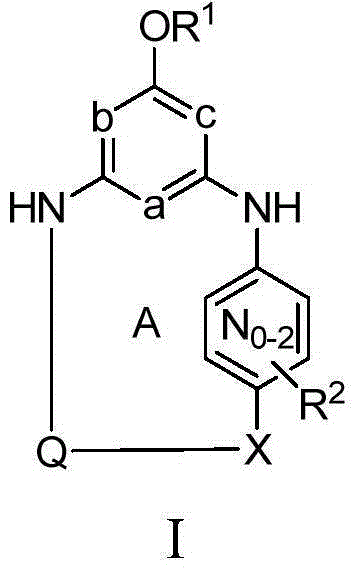
[0034] Unless expressly stated otherwise elsewhere in this application, the following terms have the following meanings. "H" means hydrogen, including isotopes thereof, such as deuterium. "Halogen" means fluorine, chlorine, bromine or iodine. "Alkyl" means a straight or branched chain alkyl group consisting of 1-6 carbons. "Alkenyl" means a straight or branched chain alkyl group consisting of 2 to 6 carbons having at least one double bond. "Cycloalkyl" means a single ring system consisting of 3-8 carbons. "Alkylene" means a straight or branched chain divalent alkyl group. "Alkenylene" means a straight or branched divalent alkyl group having at least one double bond. "Cycloalkylene" means a divalent cycloalkane moiety consisting of 3 to 7 carbons and includes gem-divalencies (eg, 1,1-cyclopropanediyl) as well as non-gem-divalencies (eg, 1 ,4-cyclohexanediyl). "Alkylidinyl" means a divalent alkene substituent in which the divalence occurs on the same carbon of the alkene. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com