New application of guanosine
A technology for guanine nucleoside and uses, applied in the field of NF-κB, STAT3 inhibitors or drugs for treating asthma, and preparation of MAPK
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
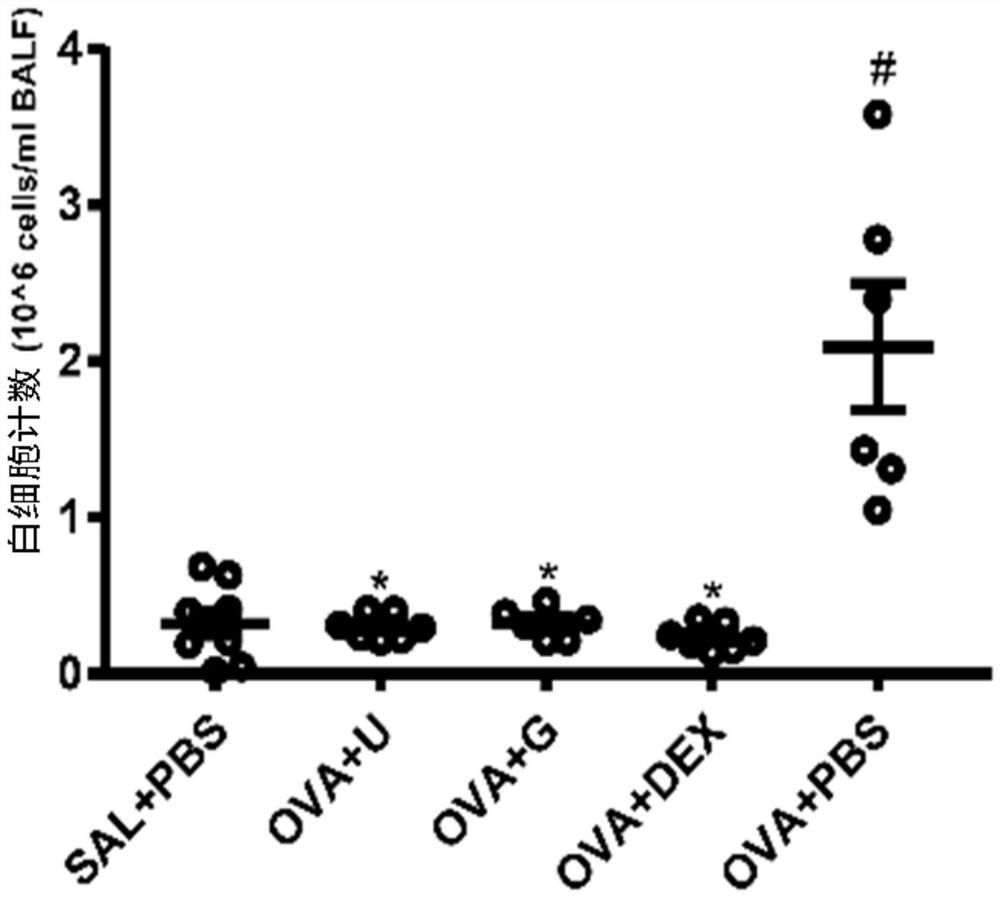
[0042] Example 1: Guanosine reduces airway inflammation in asthmatic mice induced by ovalbumin (OVA) The experimental procedure of this example is as follows:
[0043] (1) Establishment of asthma animal model
[0044] Newly purchased balb / c female mice at the age of 4-6 weeks were bred in an SPF-grade environment for one week before the experiment was carried out.
[0045] 1) On the 1st and 14th day, the negative control group was intraperitoneally injected with 100 μL of 0.9% NaCl; except the negative control group, each mouse in the other groups was intraperitoneally injected with 100 μL of a solution containing 40 μg of ovalbumin (OVA) and 4 mg of aluminum adjuvant.
[0046] 2) On the 24th to 26th day, the mice inhaled 1% OVA solution (dissolved in PBS) by ultrasonic atomization for 30 minutes, and the negative control group inhaled the nebulized PBS buffer solution for 30 minutes. One hour before nebulization, the dexamethasone group was intraperitoneally injected with 1 ...
Embodiment 2
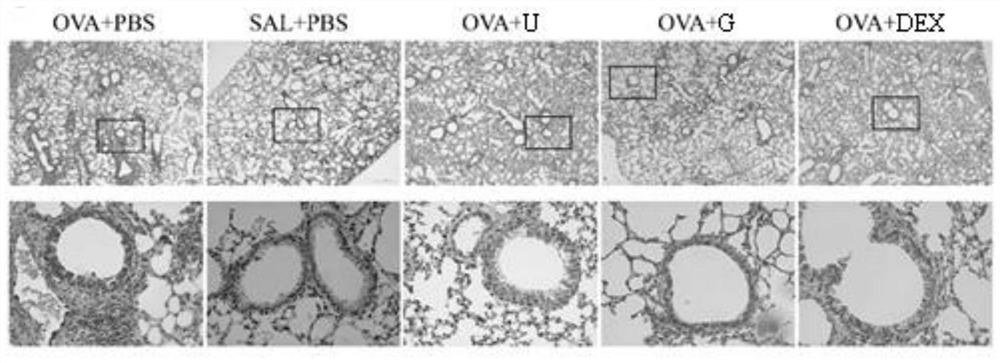
[0063] Example 2: Guanine nucleoside alleviates airway fibrosis in asthmatic mice induced by ovalbumin (OVA)
[0064] In this example, an animal model of asthma was established in the same manner as in Example 1.
[0065] Then perform Masson staining:
[0066] On day 27, after the detection of airway hyperresponsiveness, the left lung lobe was placed in an embedding frame, fixed with 4% paraformaldehyde, routinely dehydrated and embedded, sectioned at a thickness of 4 μm, and then stained according to the following steps:
[0067] 1) Dewaxing: xylene (I) 10min, xylene (II) 10min
[0068] 2) Rehydration: Soak in 100%, 95%, 80%, and 60% in turn for 5 minutes; wash with double distilled water 3 times, 3 minutes each time;
[0069] 3) Weigert hematoxylin staining for 8 minutes;
[0070] 4) Differentiate with acidic ethanol for 5 seconds, wash with distilled water for 1 minute;
[0071] 5) Masson blue solution was turned blue for 3 minutes, and washed with distilled water for 1...
Embodiment 3
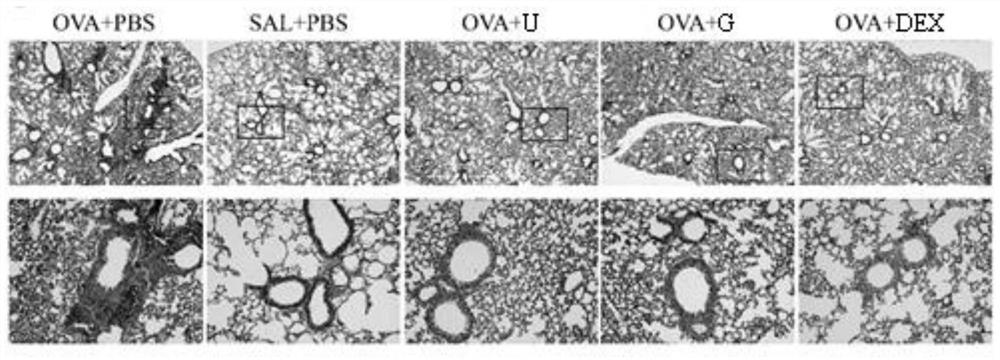
[0079] Example 3: Guanosine significantly reduces airway hyperresponsiveness in asthmatic mice
[0080] Airway hyperresponsiveness is a major pathological feature of asthma. In this embodiment, the whole plethysmography system is used to measure the enhanced expiratory pause value (Penh) of mice after being challenged with different concentrations of acetylcholine chloride. The specific process is as follows:
[0081] In this example, an animal model of asthma was established in the same manner as in Example 1. On the 27th day, the mice were placed in the body scanning box of the whole plethysmography system to cool down for 10 minutes, and then given 0, 12.5, 25, 50, 100 mg / mL acetylcholine chloride solution for 2 min, and after each atomization, Record the Penh value within 6 minutes.
[0082] The result is as Figure 4 As shown, the Penh value of the mice in the asthma model group was significantly higher than that of the negative control group; compared with the model gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com