Compound extracted from symbiotic fungus Paracoiothyrium brasiliense and application of compound
A technology for symbiotic fungi, compounds, applied in the separation/purification of carbonyl compounds, microorganisms, microorganism-based methods, etc., can solve problems such as drug resistance, high bleeding risk, and low cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]A method for the preparation of four new compounds derived from a class of symbiotic fungi, the four new compounds are isolated from the fermentation broth of Paraconiothyrium brasiliense strains isolated from the intestines of the Chinese sword-horned locust in the Shennongjia area .
[0041] The Paraconiothyrium brasiliense strain was preserved in the China Center for Type Culture Collection (Wuhan University) on May 25, 2016, address: Luojia Mountain, Wuchang, Wuhan City, Hubei Province, and the preservation name is: Cyclospora brasiliensis MZ-1, The classification name is Paraconiothyrium brasiliense MZ-1, and the deposit number is CCTCC M2016279.
[0042] The concrete preparation method of described 4 compounds is as follows:
[0043] Insert the symbiotic fungus Paraconiothyrium brasiliense frozen into the PDA solid medium for recovery, cultivate for 3 days to obtain the seed bacterial block, select the seed bacterial block of the size of 0.5cm * 0.5cm to insert in...
Embodiment 2
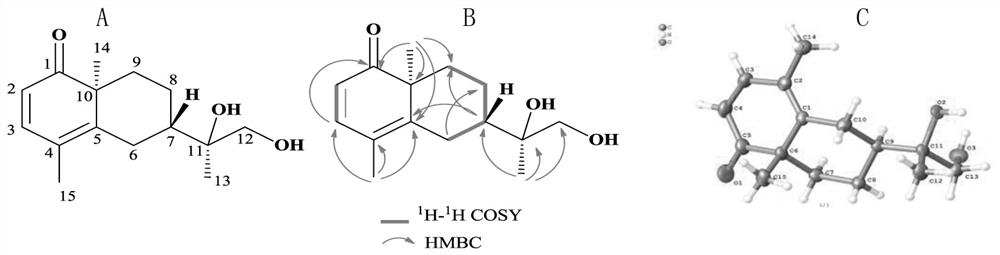
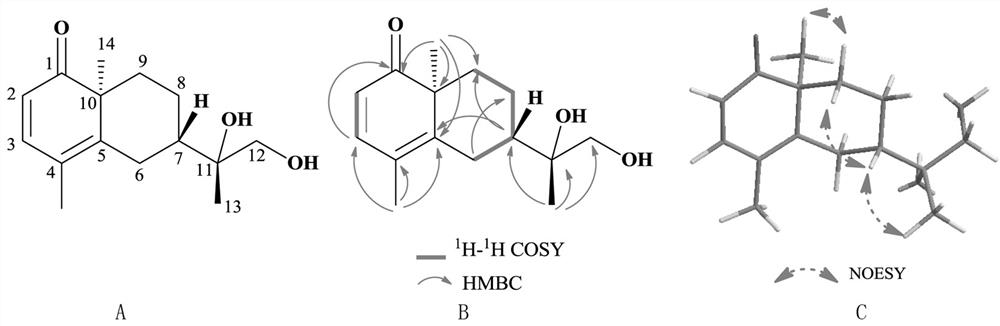
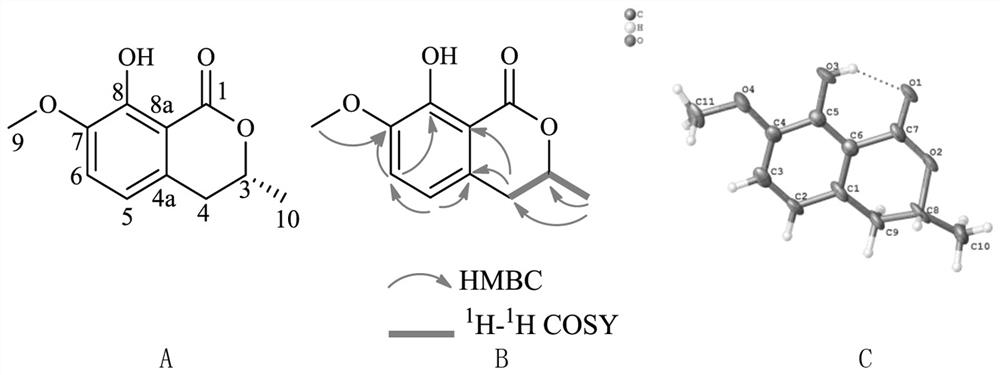
[0050] Compound 1: Crystalline (ethyl acetate). By high resolution mass spectrometry HR-ESI-MS m / z:251.1646[M+H]+(C 15 h 23 o 3 , calcd.251.1647), determine its molecular formula as C 15 h 22 o 3 , with an unsaturation of 5. Compound 1 1 H-NMR spectrum (Table 1) shows 2 alkenes proton signal δ H 5.89 (1H, d, J=9.8Hz, H-2), 7.08 (1H, d, J=9.8Hz, H-3), 4 methylene proton signals δ H 1.98(1H, t, J=12.9Hz, H-6a), 2.74(1H, d, J=12.9Hz, H-6b), 1.51(1H, m, H-8a), 1.59(1H, m, H -8b), 1.17 (1H, dd, J=12.2, 3.7, Hz H-9a), 1.89 (1H, t, J=3.1Hz, H-9b), 3.22 (1H, d, J=10.8Hz, H -12a), 3.26 (1H, d, J=10.8Hz, H-12b), 3 methyl proton signals δ H 1.02 (3H, s, H-13), 1.13 (3H, s, H-14), 1.90 (3H, s, H-15). Compound 1 13 In the C-NMR spectrum (Table 1), HSQC spectrum and DEPT spectrum, there are 15 carbon signals, including a carbonyl carbon signal δ C 206.4(C-1); four olefinic carbon signals δ C 122.2(C-2), 148.9(C-3), 120.7(C-4) and 153.3(C-5); two oxycarbon signals δ C 73.4 (C-...
Embodiment 3
[0066] Anti-platelet aggregation activity of 4 compounds obtained according to Example 1
[0067] In order to evaluate the anti-platelet aggregation activity of the compounds, some compounds were screened and compared by direct anti-platelet aggregation inhibitor chromogenic assay. Direct anti-platelet aggregation inhibitor chromogenic assay method: with aspirin as positive drug, positive drug and compound are dissolved with 5% DMSO physiological saline solution, select appropriate concentration (1mmol / L) sample to measure, experiment is set up experimental group and blank group, Mix the fresh plasma with sodium citrate (9:1, V / V), centrifuge at 1500g for 10 minutes to obtain the supernatant, preheat it in a 37°C incubator, take 50 μL of the compound and 450 μL of the plasma supernatant and mix well, After reacting at 37° C. for 5 minutes, 20 μL of ADP (10 μmol / L) was added, and the OD value was measured at a wavelength of 405 . Three parallel operations were performed, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com