Pharmaceutical composition and its preparation
A technology of composition and medicine, which is applied in the field of medicine, can solve problems such as the easy oxidation of guanosine nucleoside, and achieve the effect of good therapeutic effect, controllable quality and clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
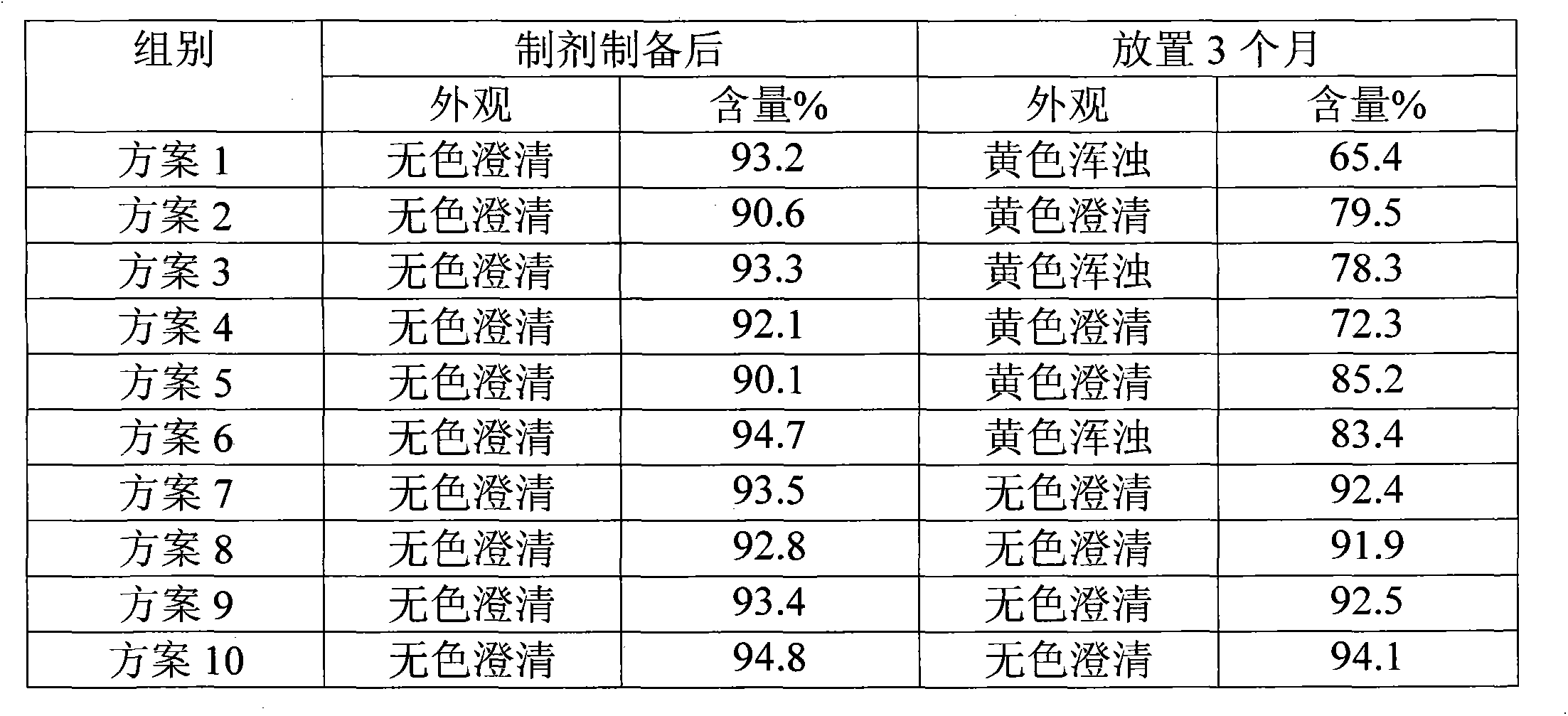
Image
Examples
Embodiment 1
[0061] prescription:
[0062] Element
[0063] Preparation method: Take 100L of water for injection, heat to boil, vacuumize, fill with nitrogen, put in raw and auxiliary materials at a water temperature of 70°C-90°C: proline, serine, alanine, isoleucine, leucine, tyrosine , glutamic acid, phenylalanine, arginine, lysine, valine, threonine, glycine, after being completely dissolved, add sorbitol, and lower the temperature at the same time, when the temperature of the liquid drops to 60°C, add the composition Amino acid, when the temperature of the medicinal solution is lowered to 45°C, add cysteine, after it is completely dissolved, add the prescribed amount of guanosine and stir until completely dissolved, add water for injection to the specified amount, stir evenly, 0.45μm filter element, 0.22 Filter with a μm filter element, fill the filtrate into a non-PVC composite co-extruded film infusion bag under nitrogen protection, seal it, and sterilize at 115°C for 30 m...
Embodiment 2
[0065] prescription:
[0066] Element
[0067] Preparation method: Take 100L of water for injection, heat to boil, vacuumize, fill with nitrogen, put in raw and auxiliary materials at a water temperature of 70°C-90°C: proline, serine, alanine, isoleucine, leucine, aspartic acid Acid, tyrosine, glutamic acid, phenylalanine, arginine, lysine, valine, threonine, glycine, after being completely dissolved, add sorbitol, and cool down at the same time, until the temperature of the liquid drops to 60 ℃, add histidine, and when the temperature of the liquid drops to 45 ℃, add cysteine, and after it is completely dissolved, add the prescribed amount of guanosine and stir until completely dissolved, add water for injection to the specified amount, and stir evenly. Filter with a 0.45 μm filter element and a 0.22 μm filter element, fill the filtrate into a non-PVC composite co-extruded film infusion bag under nitrogen protection, seal it, and sterilize at 115°C for 30 minutes t...
Embodiment 3
[0069] prescription:
[0070] Element
[0071] Preparation method: Take 100L of water for injection, heat to boil, vacuumize, fill with nitrogen, put in raw and auxiliary materials at a water temperature of 70°C-90°C: proline, serine, alanine, isoleucine, leucine, aspartic acid Acid, tyrosine, glutamic acid, phenylalanine, arginine, lysine, valine, threonine, glycine, cystine, add sorbitol after completely dissolved, cool down at the same time, wait for the drug When the temperature of the liquid drops to 60°C, add histidine, when the liquid temperature drops to 45°C, add cysteine, add the prescribed amount of guanosine and stir until completely dissolved, add water for injection to the specified amount , stir evenly, filter with a 0.45 μm filter element and a 0.22 μm filter element, fill the filtrate into a non-PVC composite co-extruded film infusion bag under nitrogen protection, seal it, and sterilize at 115°C for 30 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com