Preparation method of acyclovir
A technology of diacetyl acyclovir and a synthesis process, applied in the field of organic synthesis, can solve the problems affecting the product yield stability and product quality, the synthesis process is difficult to industrialize production, and the product separation and purification is difficult, etc. The effect of purification and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
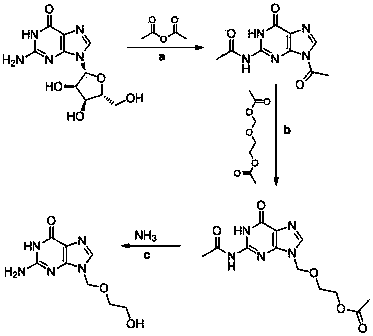
[0023] a) Synthesis of diacetylguanine
[0024] Add guanosine, acetic anhydride, and boric acid into the reaction kettle at a weight ratio of 1:6.4:0.006, raise the temperature to 110°C-120°C, and keep the temperature for 6 hours. Then the temperature was lowered to 100° C. for 6 hours and the reaction was completed. Part of the reaction solution (40% of the feed amount) was distilled off under reduced pressure. Lower the temperature to 5°C and keep it for 5 hours, discharge and centrifuge, wash with acetic anhydride three times, and dry to obtain diacetylguanine. 1 H NMR (400 MHz,
[0025] DMSO): δ =8.45(s, 1H, Ar-H), 2.81(s, 3H, CH 3 ), 2. 21 (s, 3H, CH 3 ); 13 C NMR (DMSO, 100 MHz): δ = 173.4, 168.0, 154.4, 148.3, 147.6, 137.4, 24.6, 24.0.
[0026] b) preparation of diacetyl acyclovir
[0027] Put toluene, diacetylguanine, and aluminum trichloride catalysts into the reaction kettle in proportion, slowly raise the temperature to reflux, separate water, add 2-oxa-...
Embodiment 2
[0031] b) The catalyst used is anhydrous zinc chloride and benzenesulfonic acid, and the weight ratio of the two is 1:2. Others are the same as the above-mentioned embodiment. Obtained content greater than 99.5% acyclovir bulk drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com