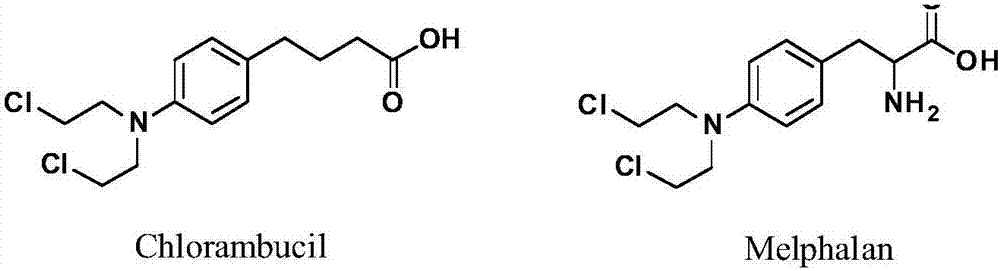
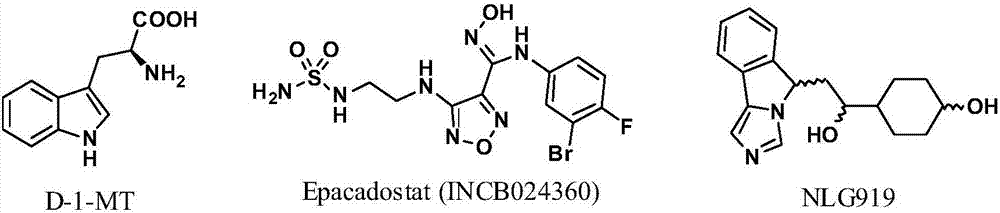
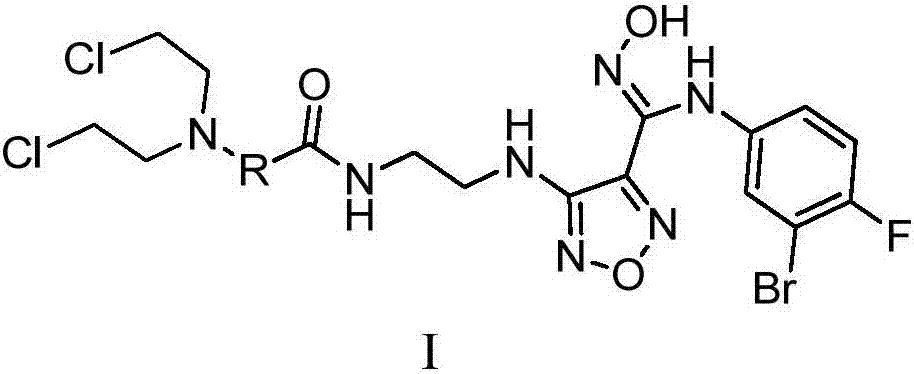
Targeted indoleamine-2,3-dioxygenase 1 nitrogen mustard inhibitor as well as preparation method and application thereof
A compound and amino technology, applied in the field of medicine, can solve the problems of unreported research on multi-target inhibitors, achieve good IDO1 inhibitory activity, delay tumor growth, and broad-spectrum anti-tumor activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: 3-(bis(2-chloroethyl)amino)-N-(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxycarboxamidino) Synthesis of -1,2,5-oxadiazole-3-methyl)amino)ethyl)benzamide
[0053] A, 3-(bis(2-chloroethyl)amino)-N-(2-((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro -1,2,4-oxadiazole-3-methyl)-1,2,5-oxadiazole-3-methyl)amino)ethyl)benzamide
[0054] Compound 3-(4-((2-aminoethyl))amino-1,2,5-oxadiazole-3-methyl)-4-(3-bromo-4-fluorophenyl)-1,2 , 4-oxadiazol-5(4H)-ketone hydroiodide (0.20g, 0.4mmol) and 3-(bis(2-chloroethyl)amino)benzoic acid (0.11g, 0.4mmol) were dissolved in DMF HATU (0.15g, 0.4mmol) and DIPEA (0.18mL, 1mmol) were added, and after stirring overnight at room temperature, the reaction solution was poured into ice water, extracted with ethyl acetate, washed with saturated aqueous sodium chloride, and dried over anhydrous sodium sulfate. The crude product was concentrated and purified by silica gel column chromatography (eluent: dichloromethane / methanol=120:1...
Embodiment 2
[0057] Example 2: 2-(3-(bis(2-chloroethyl)amino)phenyl)-N-(2-((4-(N-(3-bromo-4-fluorophenyl)-N' Synthesis of -Hydroxyformamido)-1,2,5-oxadiazole-3-methyl)amino)ethyl)acetamide
[0058] According to the method of Example 1, in step A, replace 3-(bis(2-chloroethyl)amino)benzoic acid with 2-(3-(bis(2-chloroethyl)amino)phenyl)acetic acid, in In Step B, 2-(3-(bis(2-chloroethyl)amino)phenyl)-N-(2-((4-(4-(3-bromo-4-fluorophenyl)-5- Carbonyl-4,5-dihydro-1,2,4-oxadiazole-3-methyl)-1,2,5-oxadiazole-3-methyl)amino)ethyl)acetamide instead of 3- (Bis(2-chloroethyl)amino)-N-(2-((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2 , 4-oxadiazole-3-methyl)-1,2,5-oxadiazole-3-methyl)amino)ethyl)benzamide to obtain 2-(3-(bis(2-chloroethyl )amino)-N-(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxycarboxamidino)-1,2,5-oxadiazole-3-form Base) amino) ethyl) acetamide 0.12g, yield 77%.
Embodiment 3
[0059] Example 3: 3-(3-(bis(2-chloroethyl)amino)phenyl)-N-(2-((4-(N-(3-bromo-4-fluorophenyl)-N' Synthesis of -Hydroxyformamido)-1,2,5-oxadiazole-3-methyl)amino)ethyl)propionamide
[0060] According to the method of Example 1, in step A, replace 3-(bis(2-chloroethyl)amino)benzoic acid with 3-(3-(bis(2-chloroethyl)amino)phenyl)propionic acid, In step B with 3-(3-(bis(2-chloroethyl)amino)phenyl)-N-(2-((4-(4-(3-bromo-4-fluorophenyl)-5 -carbonyl-4,5-dihydro-1,2,4-oxadiazole-3-methyl)-1,2,5-oxadiazole-3-methyl)amino)ethyl)propionamide instead of 3 -(bis(2-chloroethyl)amino)-N-(2-((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1, 2,4-oxadiazole-3-methyl)-1,2,5-oxadiazole-3-methyl)amino)ethyl)benzamide gives 3-(3-(bis(2-chloroethyl Base)amino)phenyl)-N-(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxycarboxamidino)-1,2,5-oxadiazole -3-methyl)amino)ethyl)propionamide 0.23g, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com