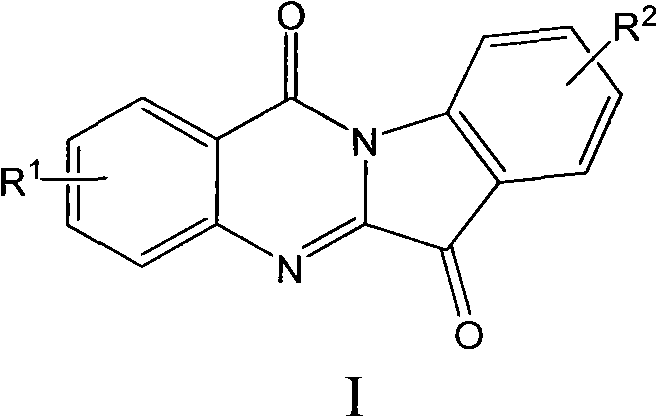
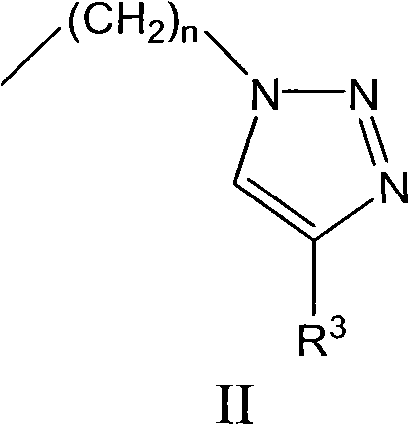
Novel indoleamine-2,3-dioxygenase inhibitor as well as preparation method and application thereof
A technology of polymorphism and triethylamine, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., to achieve the effects of easy industrial production, high yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
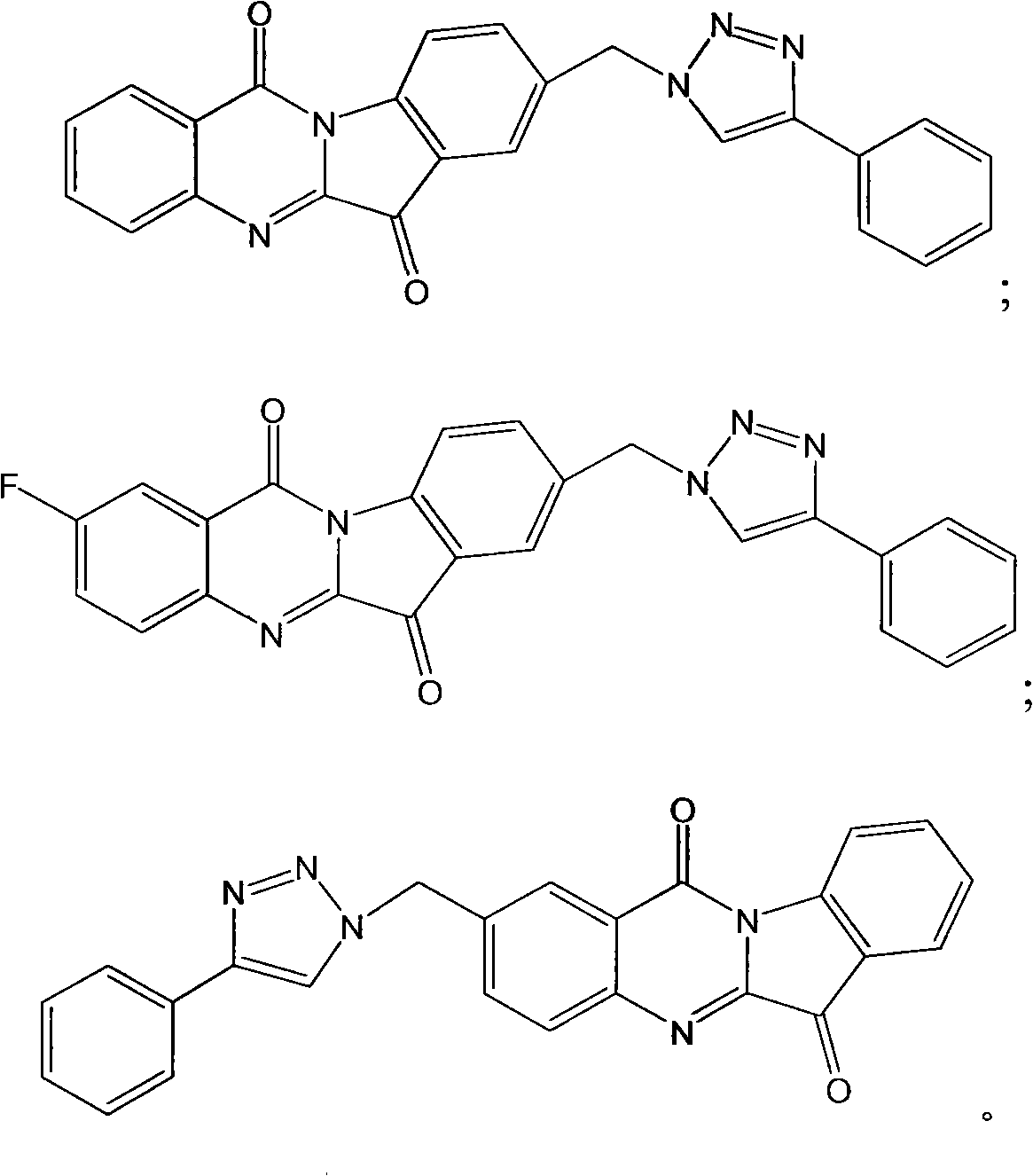
[0101] Example 1: 8-(4-phenyl-1H-1,2,3-triazol-1-ylmethyl)tryptanthrin
[0102] (1) Preparation of 1-azidomethyl-4-nitrobenzene
[0103]
[0104] Add p-nitrobenzyl chloride (162mg, 1mmol), sodium azide (120μL, 1.2mmol), and dimethyl sulfoxide (121mg, 1.2mmol) into the reaction flask in sequence, and keep the aluminum foil away from light, and react at room temperature for 0.5-1h. TLC detection showed that after the reaction was completed, water was added to the reaction system, extracted with ethyl acetate, the organic phase was washed with water, dried over anhydrous sodium sulfate, and ethyl acetate was removed by vacuum rotary evaporation to obtain a light yellow-brown liquid, which was directly used in the next step reaction;
[0105] (2) Preparation of 1-(4-nitrobenzyl)-4-phenyl-1H-1,2,3-triazole
[0106]
[0107] 1-azidomethyl-4-nitrobenzene (178mg, 1mmol), sodium ascorbate (79mg, 0.4mmol), cuprous iodide (38mg, 0.2mmol), acetonitrile (3mL), water (0.3mL) and Ph...
Embodiment 2
[0124] Example 2: 2-fluoro-8-(4-phenyl-1H-1,2,3-triazol-1-ylmethyl)tryptanthrin
[0125]
[0126] The isatin derivative (304 mg, 1 mmol) containing 1,2,3-triazole structure prepared in step (5) of Example 1 and 5-fluoroisatic anhydride (purchased from Yancheng Medico Chemical Manufacturing Co., Ltd. ) (181mg, 1mmol) was added to the reaction flask, then triethylamine and toluene were added, the temperature was raised to 110°C, and the reaction was stirred under reflux for 4h. After recrystallization, 275 mg of a dark yellow-green solid was finally obtained, with a yield of 65%.
[0127] The characterization data are as follows: 1 H-NMR (400MHz, CDCl 3 ): δ=8.65(d, 1H), 8.07(m, 2H), 7.82(m, 5H), 7.57(m, 1H), 7.43(t, 2H), 7.35(t, 1H), 5.69(s, 2H).
Embodiment 3
[0128] Example 3: Preparation of 8-fluoro-2-(4-phenyl-1H-1,2,3-triazolylmethyl)tryptanthrin
[0129] (1) Synthesis of isatoic anhydride containing triazole structure
[0130]
[0131] Add 0.42mL of glacial acetic acid and 0.42mL of acetic anhydride to a 25mL reaction flask, then add (152mg, 0.5mmol) indole quinone (see Example 1) containing a triazole structure in batches, and heat the reaction solution to 80- 90°C, then add chromium trioxide in batches, and continue to react for 3 hours. After TLC detection shows that the raw materials have reacted completely, cool the reaction solution to room temperature, add 1.5mL of water, filter with suction, wash the solid with a large amount of water, and drain it with an oil pump to get a green color. The solid needn't be separated and left for the next reaction.
[0132] (2) Synthesis of tryptanthrin IDO inhibitors containing 1,2,3-triazole structure at the 2-position
[0133]
[0134] Add 5-fluoroindolequinone (purchased fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com