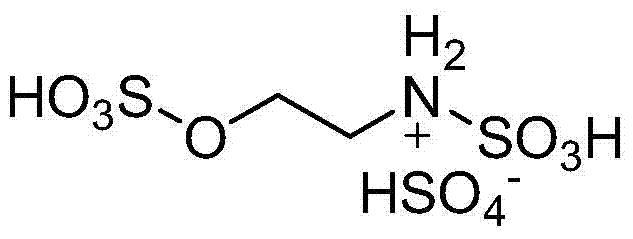
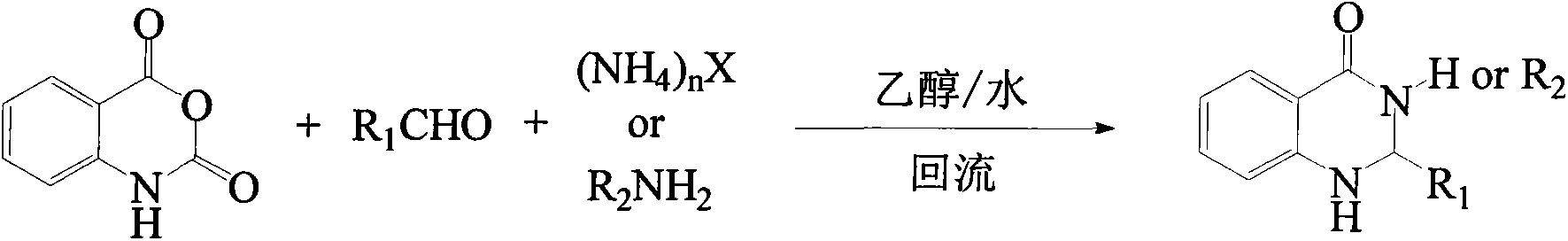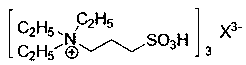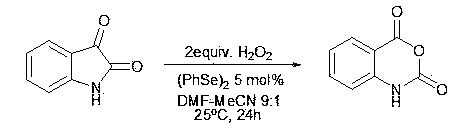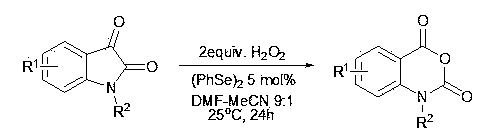Patents
Literature
95 results about "Isatoic anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
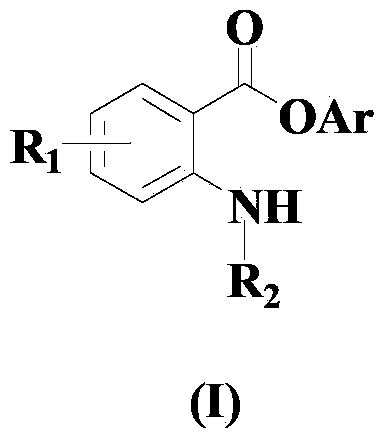
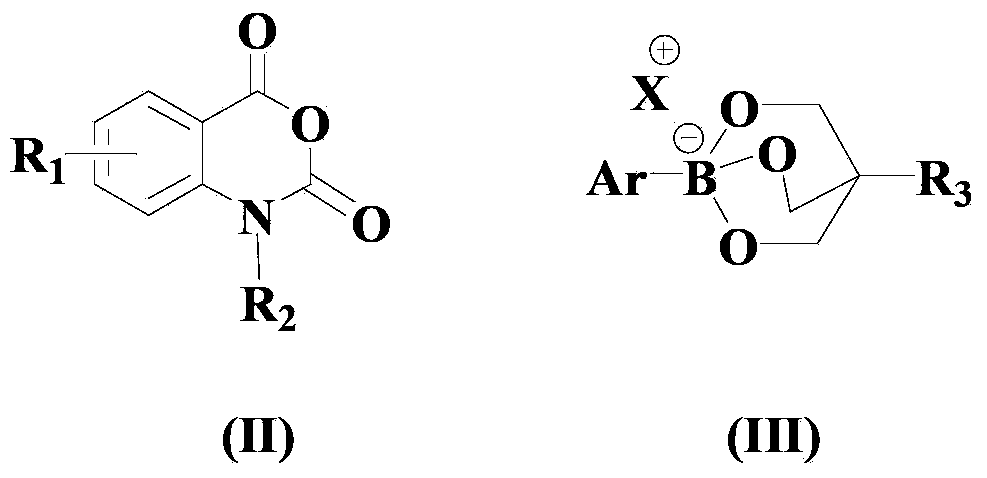
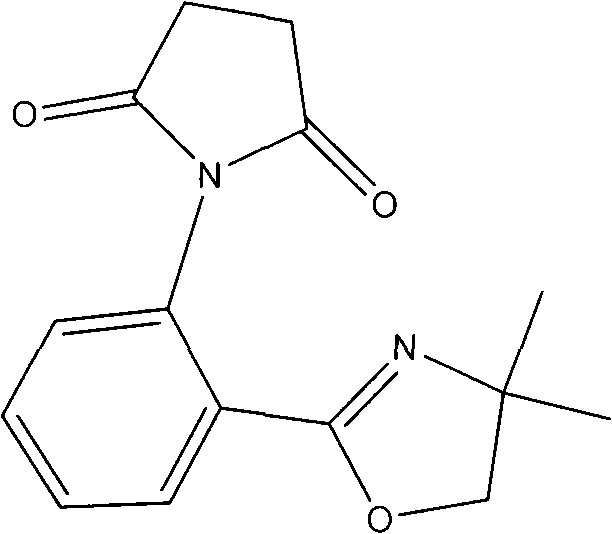
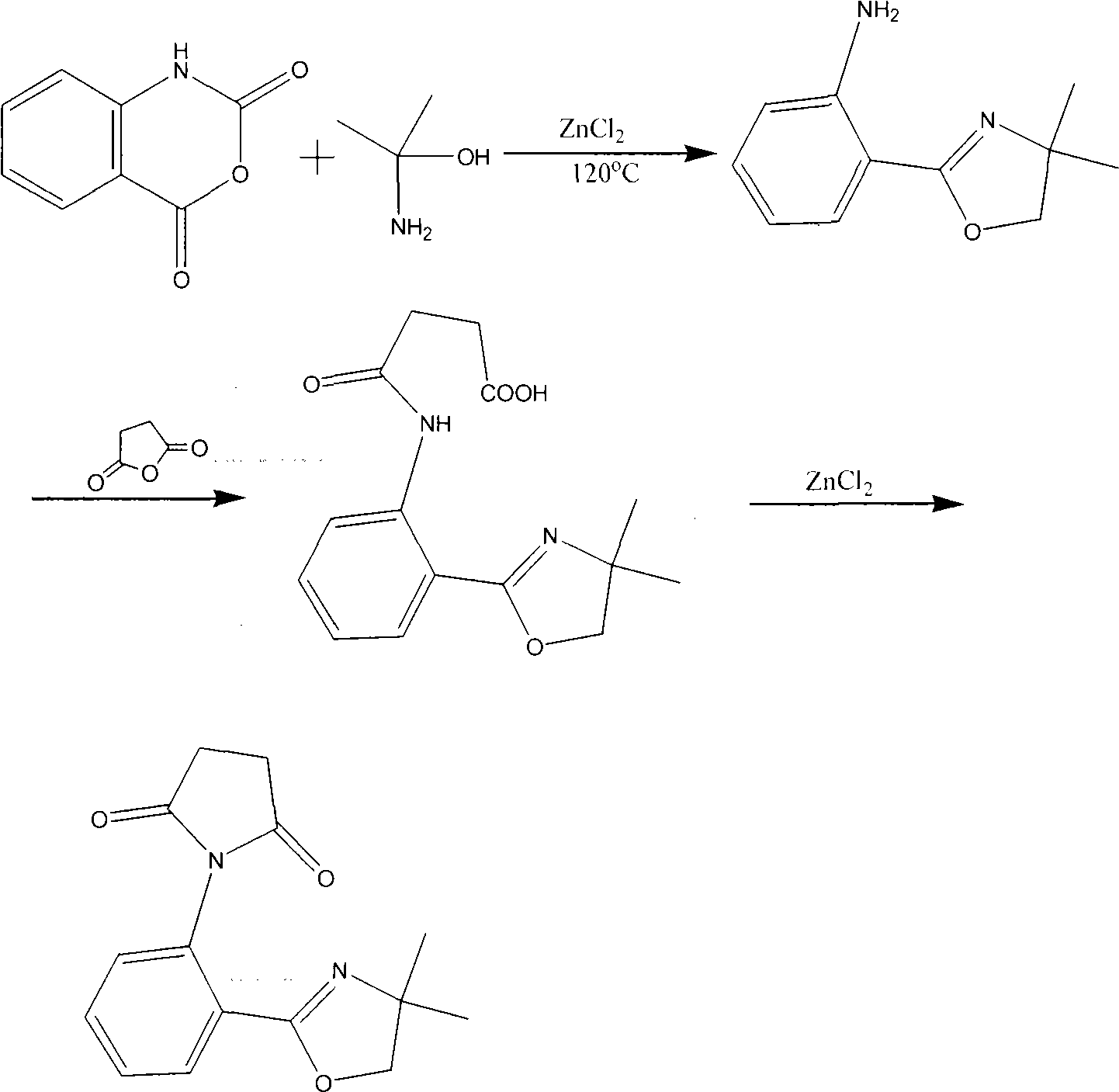
Synthesis method for ortho amino aromatic formic acid aryl ester derivatives
InactiveCN102382001AReduce dosageHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsPalladium catalyst
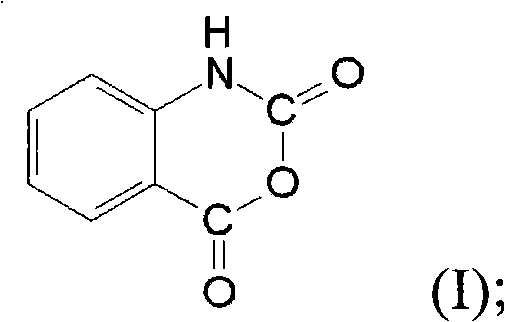
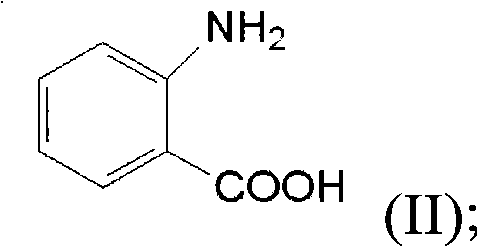
The invention discloses a synthesis method for ortho amino aromatic formic acid aryl ester derivatives as shown in the constitutional formula (I). The method includes steps of utilizing isatoic anhydride compound as shown in the constitutional formula (II) and aryl boric acid as shown in the constitutional formula (III) as raw materials to sufficiently react in inertial organic solvent in the presence of palladium catalyst, phosphine ligands and alkaline compounds, and then obtaining the ortho amino aromatic formic acid aryl ester derivatives by means of separation and purification of reaction liquid after reaction. The palladium catalyst includes one or a combination of the following palladiums: palladium chloride, palladium acetate, tetrakis (triphenylphosphine) palladium, tris (dibenzylideneacetone) dipalladium, dichloro-bis (triphenylphosphine) palladium and dichloro-bis (acetylacetonate) palladium. The synthesis method for the ortho amino aromatic formic acid aryl ester derivatives has the advantages of reasonable technology, low toxicity, mild reaction condition, high reaction yield and fine product quality.
Owner:WENZHOU UNIVERSITY
Method for compounding amino-substituted arylate compound
ActiveCN103420860AAchieving ring-opening decarboxylationHigh yieldOrganic compound preparationAmino-carboxyl compound preparationOrganic solventIsatoic anhydride
The invention relates to a method for compounding an amino-substituted arylate compound. The method comprises the step that under the condition of the adoption of a copper source compound catalyst and a ligand, and isatoic anhydride derivative and organic cyclic borate compound react in an organic solvent, so that the decarboxylation coupling reaction of isatoic anhydride compounds is realized, high-yield and high-purity amino-substituted arylate compound is obtained, a new compounding way is provided for the compounding of amino-substituted arylate compounds, and the method has good application prospects and industrial value.
Owner:JIAXING NIYA OPTOELECTRONICS CO LTD
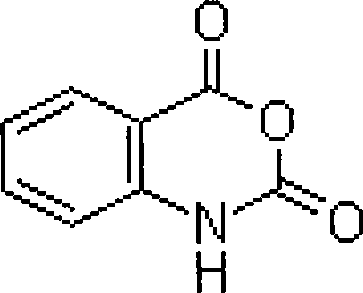
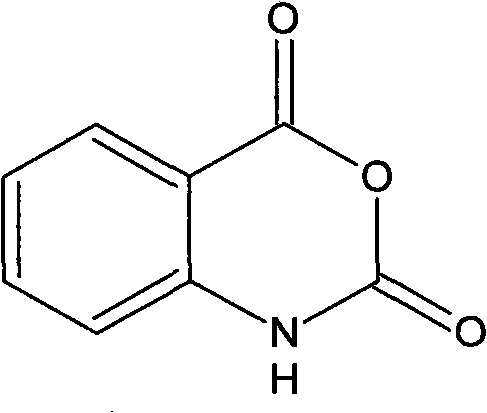
Synthesis process of isatoic anhydride
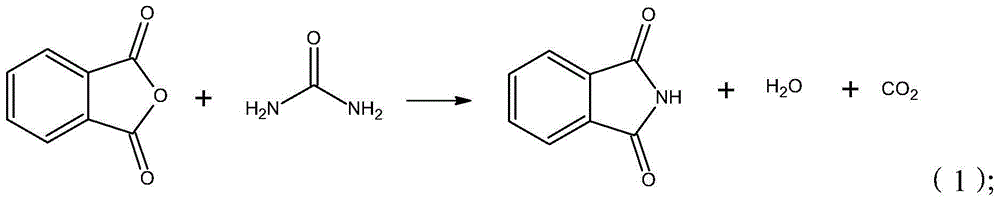
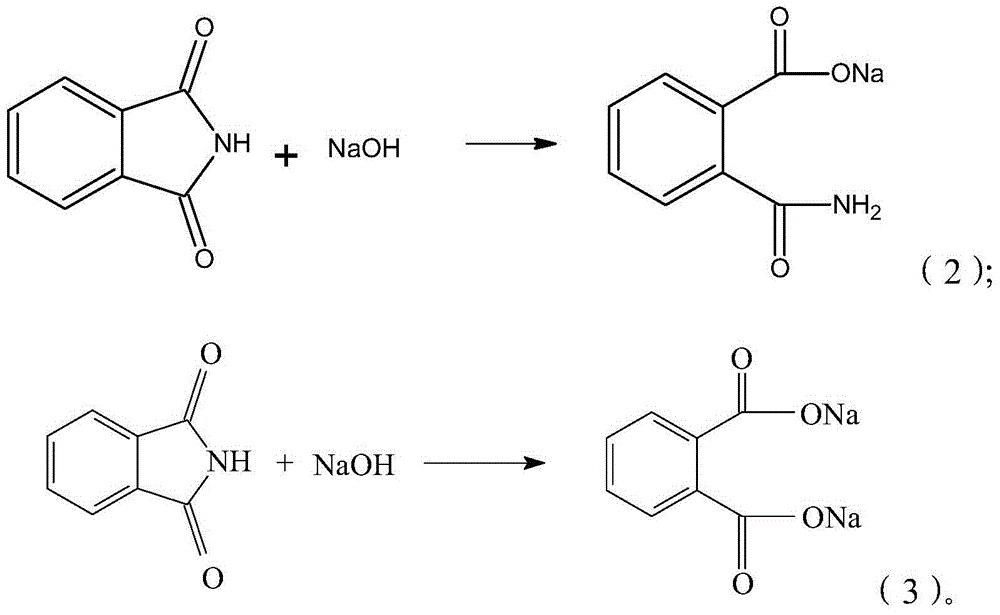
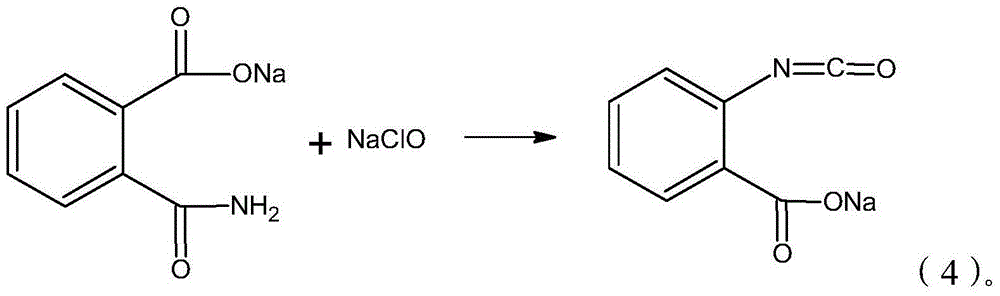
The invention provides a synthesis process of isatoic anhydride. The synthesis process comprises the following steps: a) heating phthalic anhydride until phthalic anhydride is molten and mixing the molten phthalic anhydride with urea, and cooling to 10-20 DEG C after a reaction; b) mixing reaction products obtained after cooling with liquid alkali, cooling to minus 5- 0 DEG C to carry out a first reaction, mixing reaction products with pypocholoride, mixing reaction products with hydrochloric acid after a second reaction, and carrying out a third reaction to obtain isatoic anhydride. During the synthesis process of isatoic anhydride, reaction temperature is controlled strictly. Thus, generation of by-products is avoided, and yield of isatoic anhydride is raised. Meanwhile, by one kettle way for synthesis of isatoic anhydride, industrial operations are minimized, the discharging link is reduced, and yield is raised. It shows through experimental results that yield of isatoic anhydride reaches more than 94% and purity reaches more than 99%.
Owner:甘肃西部鑫宇化学有限公司
Methods for synthesizing isatoic anhydride and N-isopropyl-2-aminobenzamide
ActiveCN101973956AResponse volume controllableHigh purityOrganic compound preparationCarboxylic acid amides preparationOrganic solvent2-aminobenzamide
The invention provides a method for synthesizing isatoic anhydride, comprising the steps of: dropwise adding a di(trichloromethyl) carbonic ester solution into an o-aminobenzoic acid solution, refluxing for reaction to obtain the isatoic anhydride. The invention further also provides a method for synthesizing N-isopropyl-2-aminobenzamide, comprising the steps of: a) dropwise adding the di(trichloromethyl) carbonic ester solution into the o-aminobenzoic acid solution, and refluxing for reaction to obtain an isatoic anhydride solution; and b) dropwise adding isopropamide into the isatoic anhydride solution, and reacting to obtain the N-isopropyl-2-aminobenzamide. After o-aminobenzoic acid and di(trichloromethyl) carbonic ester react in an organic solvent to generate the isatoic anhydride, the N-isopropyl-2-aminobenzamide can be obtained by directly adding the isopropamide for amidation, without carrying out the steps of extraction, purification, drying and the like on the isatoic anhydride, therefore, operation steps are reduced, production cycle is shortened, and industrialized production is easy to realize.
Owner:HEFEI XINGYU CHEM
Novel polyester chain extender and preparation method thereof
The invention relates to a novel polyester chain extender and a preparation method thereof. The related novel polyester chain extender can obviously improve the intrinsic viscosity of the polyester and can reduce carboxyl content in the system. The novel polyester chain extender respectively comprises o-(4,4-dimethyl oxazoline) and a butanimide structure at the ortho position of the benzene ring, wherein the imide structure can react with hydroxide radical in the polyester and can obviously improve the intrinsic viscosity of the polyester; and oxazoline can perform the chain extending reaction with carboxyl in the polyester and can reduce the carboxyl content in the system when improving the viscosity. The preparation method for the novel polyester chain extender comprises the following steps: (a) dissolving isatoic anhydride and 2-alkamine in chlorobenzene and adding a catalyst into the mixture to perform a reaction to prepare oxazoline aniline; (b) dissolving the prepared oxazoline aniline in aether, adding succinic anhydride into the mixture to perform a reaction and filtering the reaction solution to obtain an intermediate; and (c) uniformly mixing the intermediate and anhydrous zinc chloride with kieselguhr, carrying out irradiation heating on the mixture by microwave, cooling the obtained product, adding ethyl acetate into the cooled obtained product to dissolve out the product, removing the solvent and crystallizing to obtain the novel polyester chain extender.
Owner:DONGHUA UNIV
Method for synthesizing isatoic anhydride
ActiveCN101973955AEmission reductionAnhydrous reaction conditions are mildOrganic chemistryIsatoic anhydrideSolvent
The invention discloses a method for synthesizing isatoic anhydride, which comprises the following steps: (1) adding phthalimide to a reaction solvent; (2) controlling the temperature to be within the range of 40-45 DEG C, introducing chlorine gas, and reacting for 1.5-2 hours; (3) controlling the temperature to be below 45 DEG C, adding solid caustic soda, and then continuing to react for 0.5-1.5 hours; and (4) filtering and collecting salts to obtain an isatoic anhydride filtrate, cooling, crystallizing, filtering, and drying to obtain solid isatoic anhydride. The method for synthesizing the isatoic anhydride avoids the use of a large amount of virulent and dangerous chemical substances and has moderate anhydrous reaction conditions, and hydrogen chloride and sodium chloride as reaction byproducts can be treated to produce high-quality hydrochloric acid and industrial sodium chloride byproducts, thereby reducing the generation and the discharge of harmful impurities and a large amount of waste water difficult to treat. The yield can be up to 92% or above. Thus, the invention is suitable for industrial application.
Owner:HEFEI XINGYU CHEM
Synthetic method for isatoic anhydride derivative
InactiveCN102863399AMild reaction conditionsEasy to operateOrganic chemistryPolyaniline derivativesPtru catalyst
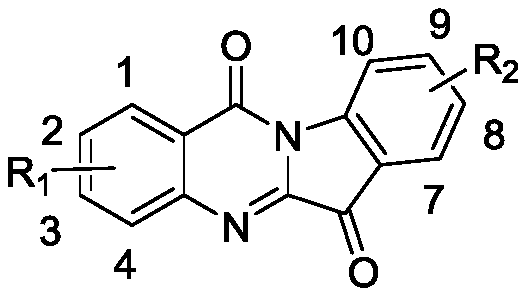
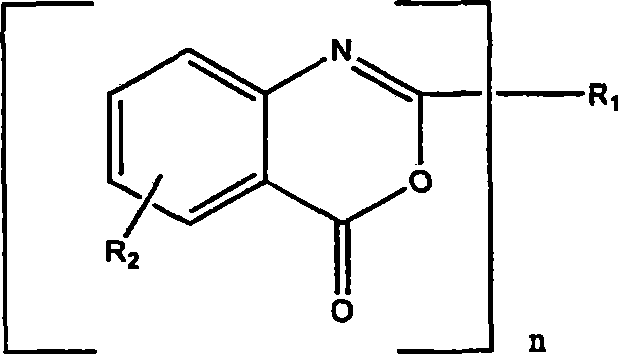
The invention discloses a synthetic method for an isatoic anhydride derivative. The method includes: reacting polyaniline derivatives shown by a formula (II) or (III) with carbon oxide with existence of Pd and Cu, or Fe and Cu metal salt catalysts in catalytic amount to obtain the isatoic anhydride derivative shown by a formula (I), wherein R1 refers to hydrogen, halogen, nitryl, alkyl, alkoxy, ester, acyl, naphthenic base, aryl or arylalkyl, and the R2 refers to hydrogen, alkyl, alkoxy, naphthenic base, aryl or arylalkyl. The synthetic method for the isatoic anhydride derivative has the advantages of mild reaction condition, simplicity in operation, wide applicable range, low production cost, high synthetic yield and the like.
Owner:NORTHWEST UNIV(CN)
Wastewater-free preparation method of bentazon
InactiveCN102617511AAvoid it happening againHigh yieldOrganic chemistryChlorosulfuric acidIsatoic anhydride
The invention belongs to the field of chemical engineering, and particularly relates to a wastewater-free preparation method of bentazon. According to the technical scheme, isatoic anhydride is used as a starting raw material, an aprotic polar solvent is used as a reaction medium in aminolysis of isopropylamine to produce amides, mixture is subjected to sulfonation of chlorosulfonic acid and cyclization, alkaline solution is directly used for extraction, and bentazon water aqua can be obtained directly. The preparation method has the beneficial effects that the yield is high, the full yield reaches above 85 percent, no wastewater is produced in the entire process, raw materials can be obtained easily, the operating is simple, and the industrialization can be realized easily.
Owner:NORTHEASTERN UNIV
Supported organic Cu(I) catalyst and preparation method and application thereof
ActiveCN107175135AOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsMicrowaveIsatoic anhydride
The invention provides a supported organic Cu(I) catalyst and a preparation method and application thereof. The preparation method provided by the invention comprises the following steps: modifying attapulgite by using a mercapto-silane coupling agent and isatoic anhydride to obtain isatoic anhydride-mercapto modified attapulgite, and enabling cuprous ions to be supported onto the isatoic anhydride-mercapto modified attapulgite through coordination to obtain the supported organic Cu(I) catalyst. The supported organic Cu(I) catalyst provided by the invention is high in catalytic activity and easy to separate, and can be repeatedly used, and supported organic Cu(I) catalyst is capable of catalyzing Glaser coupling reaction in an aqueous phase, and the reaction can be completed through short microwave heating.
Owner:深圳市冠华特化新材料有限公司
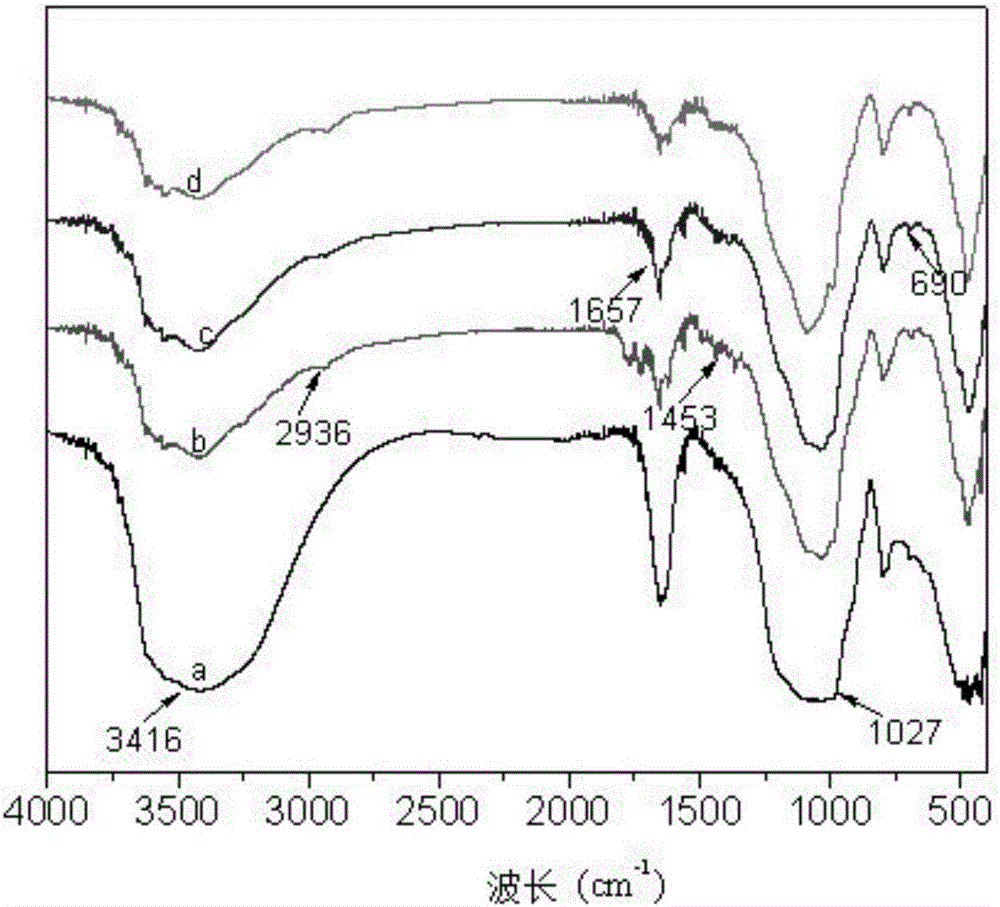
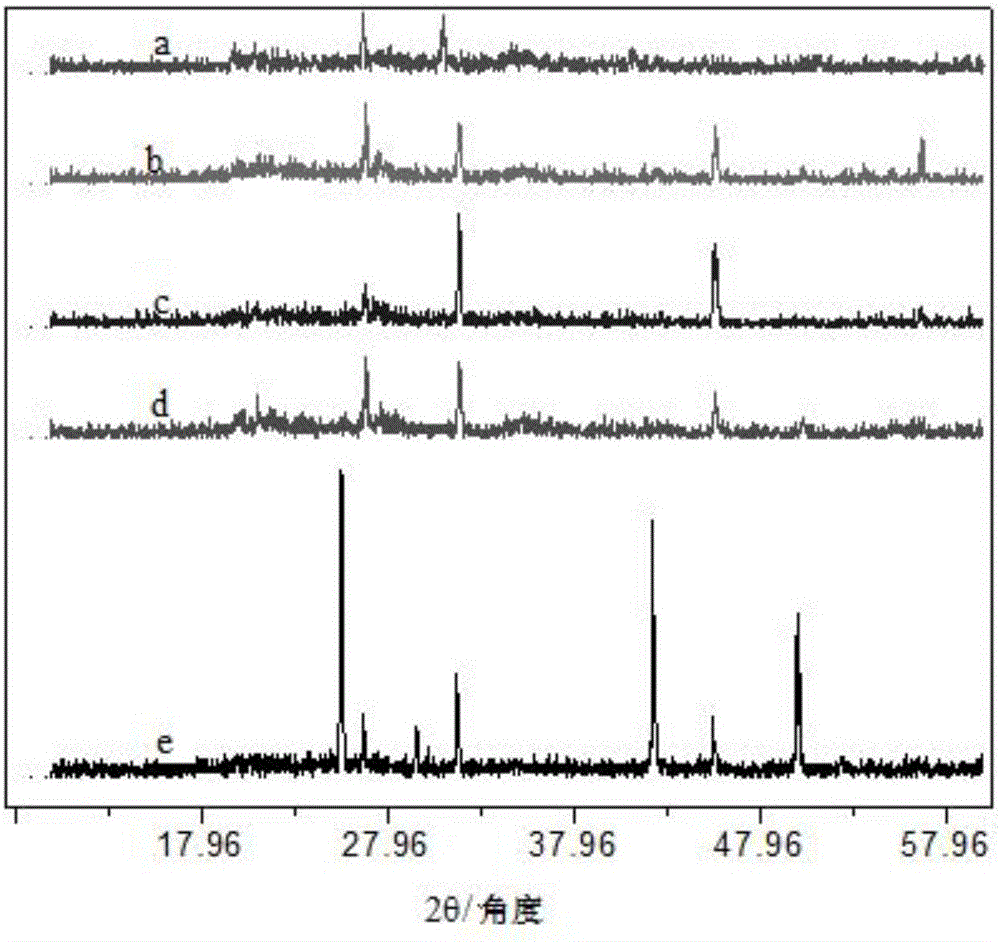
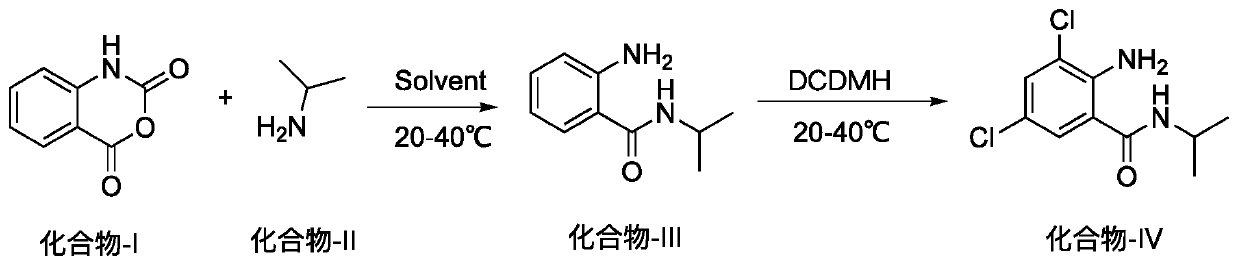
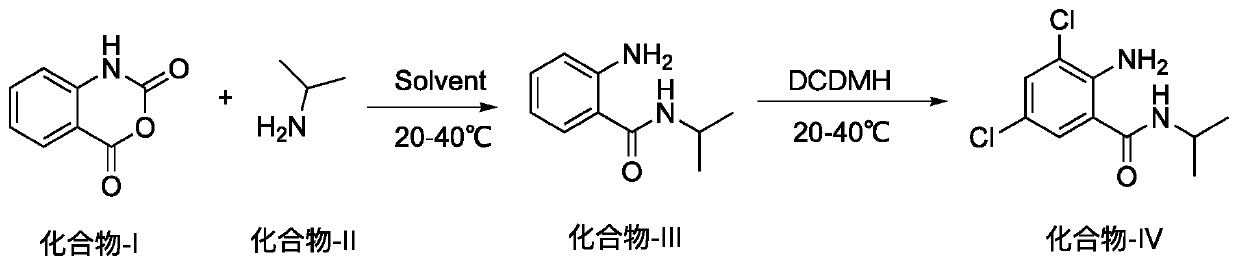
Method for preparing 2-amino-3,5-dichloro-N-isopropylbenzamide
ActiveCN110003037AHigh yieldSimple post-processingOrganic compound preparationCarboxylic acid amides preparationFiltrationReaction temperature
The invention discloses a method for preparing 2-amino-3,5-dichloro-N-isopropylbenzamide. The method comprises the following steps: (1) preparation of mixed amide: adding isatoic anhydride to a reaction solvent with the reaction concentration, which is a ratio of the solvent volume to the weight of isatoic anhydride, of 5-10 times and the reaction temperature at 20-40 DEG C, adding isopropylaminedropwise, monitoring the reaction progress in the liquid phase, and performing a reaction of a next step directly without separation after completion of the monitored reaction; and (2), preparation of2-amino-3,5-dichloro-N-isopropylbenzamide: adding dichlorohydantoin slowly to the reaction solution of the step (1), controlling the reaction temperature at 20-40 DEG C, monitoring the reaction progress in the liquid phase, performing vacuum concentration on the reaction system after completion of the reaction, performing suction filtration, washing the solid with a little hot water, and performing beating with methanol so as to obtain a white compound. According to the method for preparing 2-amino-3,5-dichloro-N-isopropylbenzamide, a novel chlorination method is adopted so as to obtain 2-amino-3,5-dichloro-N-isopropylbenzamide efficiently, and the preparation method has simple post-treatment and a high total yield.
Owner:中翌科技有限公司
ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES
The present invention provides one step processes for the preparation of substituted dibenzo[b,e][1,4]-diazepine-11-ones by the reaction of substituted isatoic anhydrides with substituted 1,2-phenylenediamines in the presence of aqueous acetic acid.
Owner:COUNCIL OF SCI & IND RES
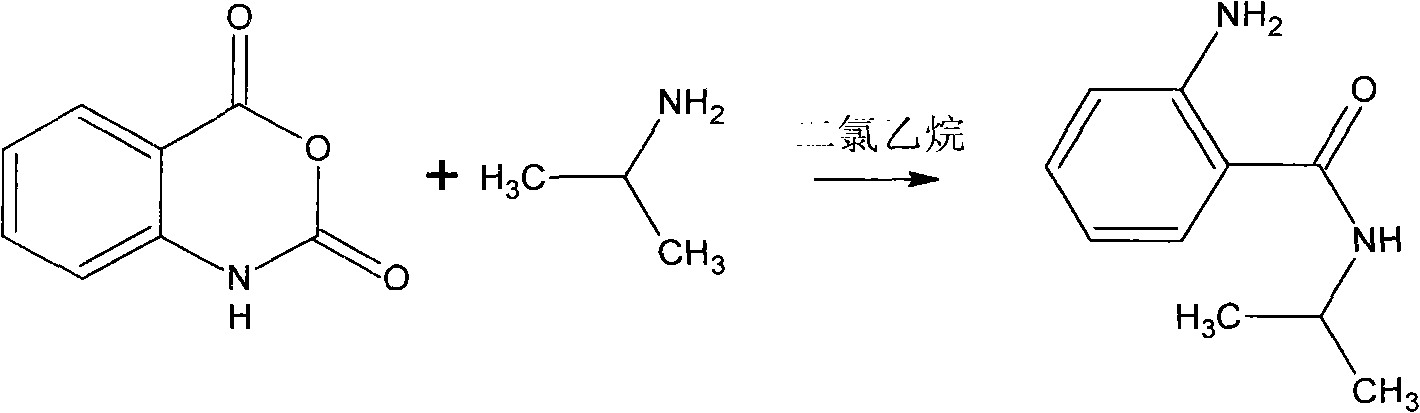
Synthesis method of bentazone midbody 2-amino-N-isopropylbenzamide
ActiveCN101967109AReduce laborAvoid harsh reaction conditionsOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsIsatoic anhydride
The invention discloses a synthesis method of a bentazone midbody 2-amino-N-isopropylbenzamide, comprising the steps of: dripping isopropylamine in a dichloroethane solution of isatoic anhydride within 3 hours at a temperature of 50-60 DEG C, continuing the reaction for 0.5-2 hours at the temperature of 50-60 DEG C, washing with water and distributing water to obtain the 2-amino-N-isopropylbenzamide, wherein the water content of the isatoic anhydride is not higher than 40% and the water content of the dichloroethane is not higher than 1%. The synthesis method of the bentazone midbody 2-amino-N-isopropylbenzamide in the invention is free from anhydrous rigorous reaction conditions to be controlled strictly, greatly decreases the production processes, lightens the labor capacity of operators, saves the energy, reduces the consumption and the production cost.
Owner:HEFEI XINGYU CHEM
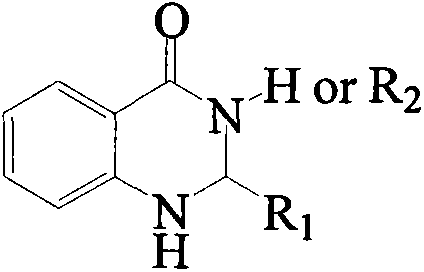
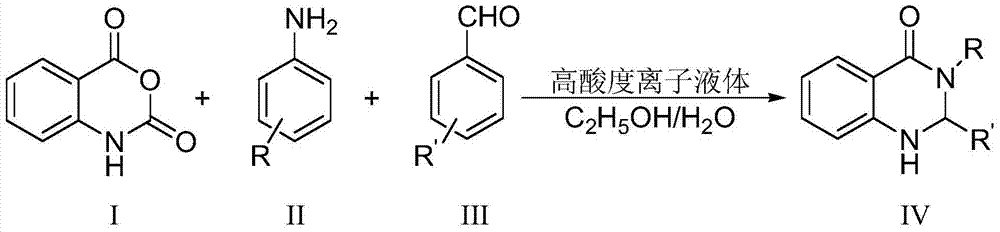
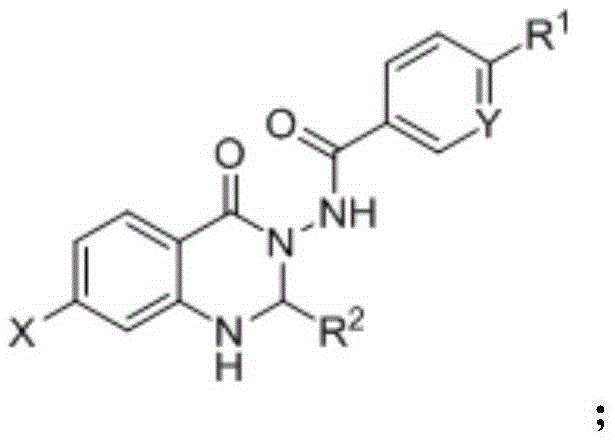
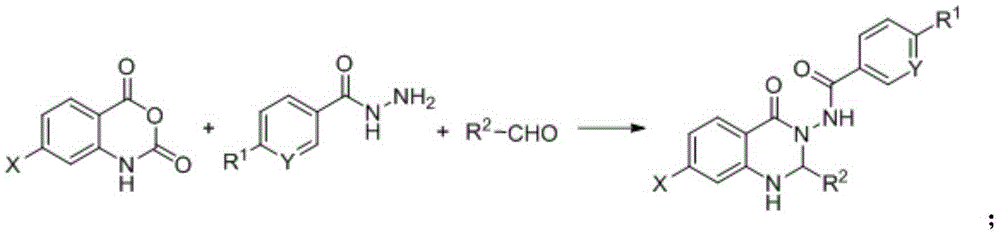
Synthesis method for substituted 2, 3-dihydro-4(1H)-quinazolinone compound
The invention discloses a synthesis method for a substituted 2, 3-dihydro-4(1H)-quinazolinone compound, and belongs to the field of medicinal preparations. According to the method, the three-component one-pot reaction of isatoic anhydride, aromatic aldehyde and ammonium salt / aromatic amine can be efficiently implemented in an ethanol / water mixed solvent without a catalyst under a reflux condition. The method has the characteristics of low cost, environment friendliness, short synthesis time and high product yield.
Owner:BOHAI UNIV
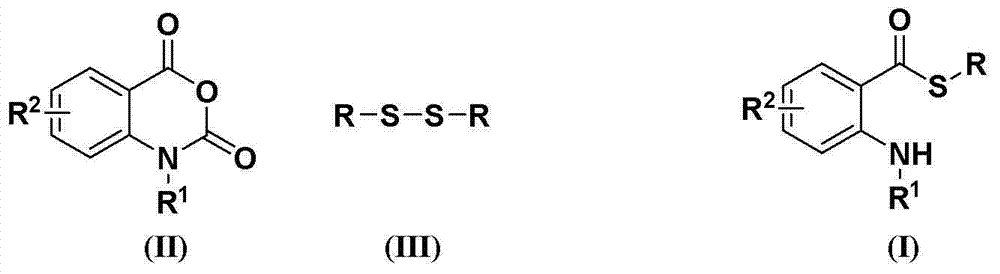
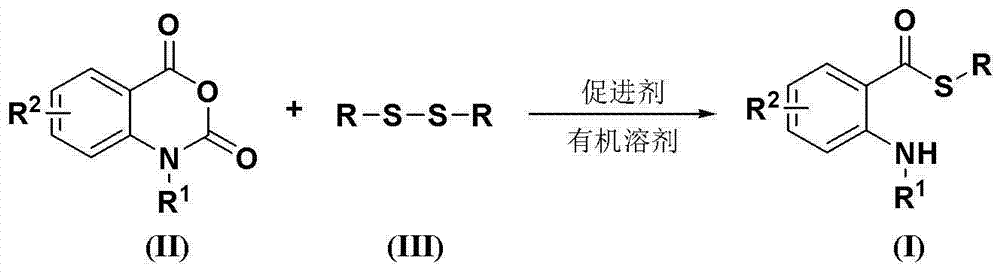
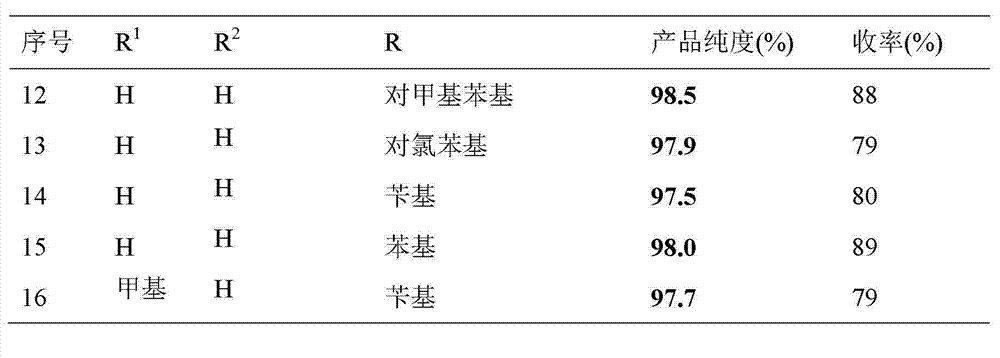
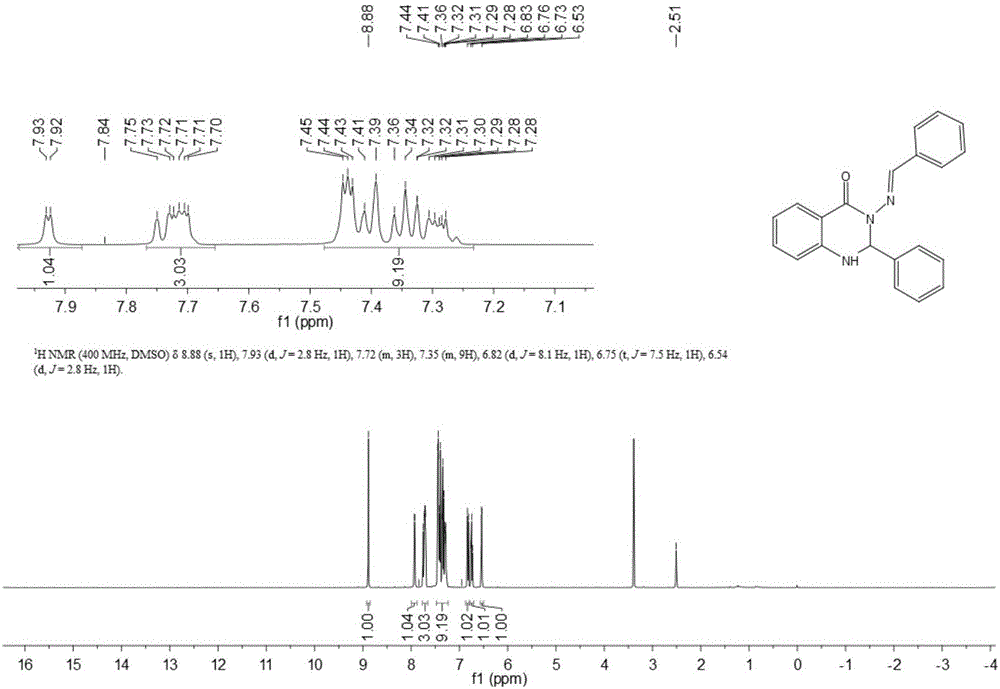
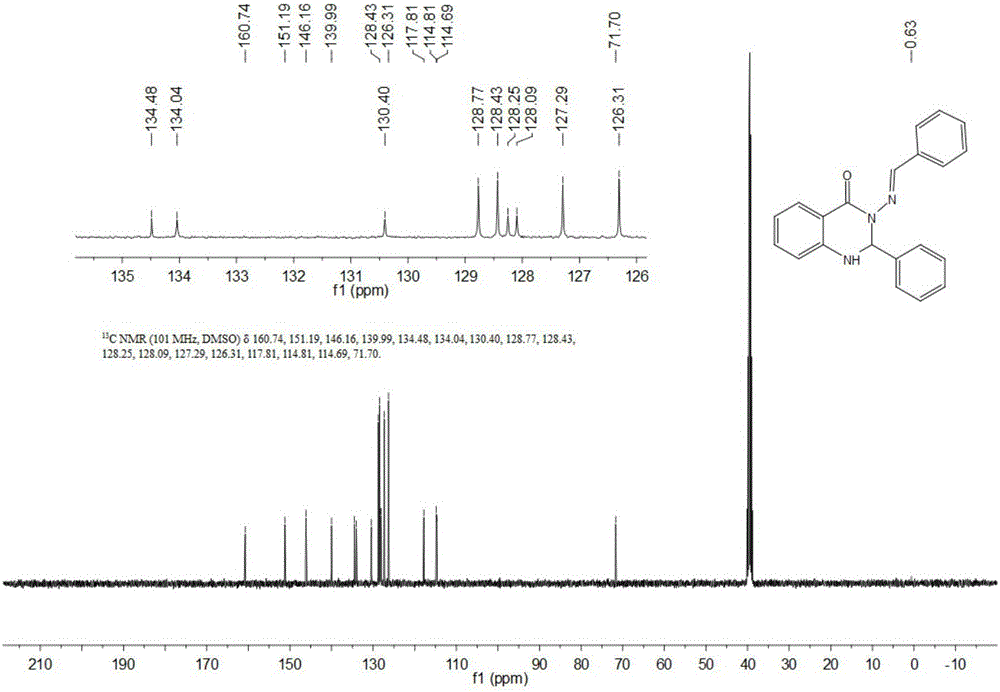
Synthesis method of S-substituted-anthranilate thioester derivatives
InactiveCN102898340AReasonable process conditionsSimple and safe operationOrganic chemistryOrganic solventSynthesis methods
The invention discloses a synthesis method of S-substituted-anthranilate thioester derivatives disclosed as Formula (I), which comprises the following steps: sufficiently reacting isatoic anhydride compounds disclosed as Formula (II) and dithioether compounds disclosed as Formula (III) in an inert organic solvent at 0-120 DEG C in the presence of an accelerator and basic compounds; and after the reaction finishes, separating and purifying the reaction solution to obtain the S-substituted-anthranilate thioester derivatives disclosed as Formula (I). The method disclosed by the invention has the advantages of reasonable technological conditions, simple and safe operation, mild reaction conditions, high reaction yield, cheap and accessible raw materials, environment-friendly substrate, low production cost and fewer three wastes.
Owner:WENZHOU UNIVERSITY
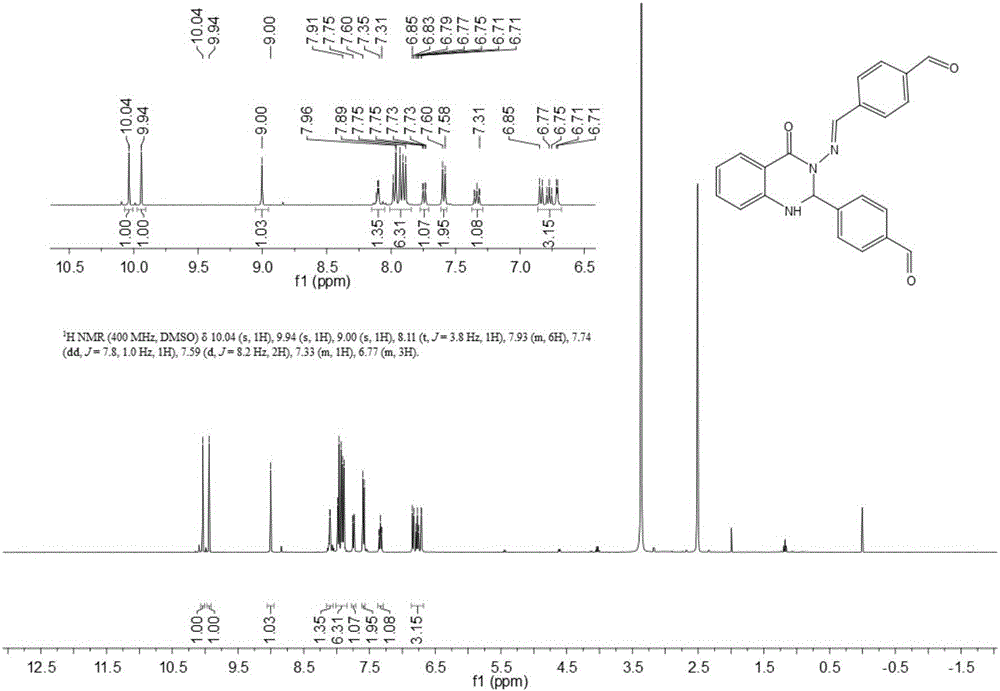
Synthetic method of quinazolinone Schiff base compounds
The invention discloses a synthetic method of quinazolinone Schiff base compounds. The synthetic method comprises the following steps: 1, raw materials including isatoic anhydride, carbonyl containing compounds and hydrazine hydrate are weighed in the mole ratio being 1:(2-2.2):1; 2, the raw materials weighed in the step 1 are mixed in a solvent, and the mixture is subjected to a heating reflux reaction with a nanometer metal oxide as a catalyst; 3, reaction products obtained in the step 2 are separated and purified, and the quinazolinone Schiff base compounds are obtained. With the adoption of the synthetic method, poisonous solvents and acid and alkaline conditions used in current synthetic reactions are avoided, reaction steps are reduced, and the reaction yield is increased.
Owner:SHAANXI UNIV OF SCI & TECH
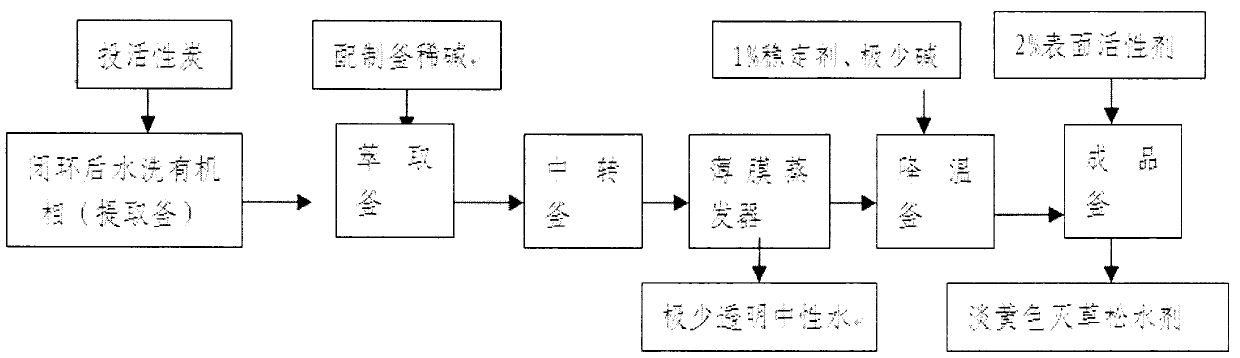
Method for preparing bentazone aqueous agent
ActiveCN102924405AEmission reductionImprove product qualityBiocideOrganic chemistryAlkaline waterClosed loop
The invention discloses a method for preparing a bentazone aqueous agent, belonging to the technical field of preparation of chemical products and aiming to provide a method for preparing a bentazone aqueous agent which is more environment-friendly and has higher appearance quality. The method comprises the following steps: taking isatoic anhydride as a raw material, carrying out amide synthesis, sulfonation synthesis and closed-loop synthesis reactions and the like in a dichloroethane solvent system, washing by water, extracting by alkaline water and concentrating by a thin-film evaporator to obtain the light-yellow bentazone aqueous agent. Compared with the traditional method, the method provided by the invention adopts less acid, base and strongly-acidic wastewater and improves the quality of the product.
Owner:甘肃西部鑫宇化学有限公司
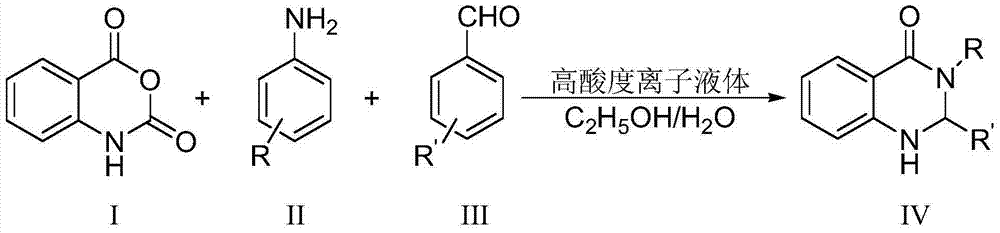
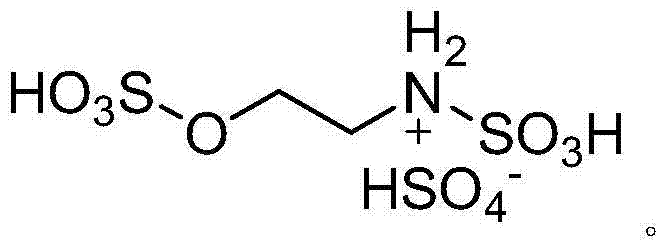
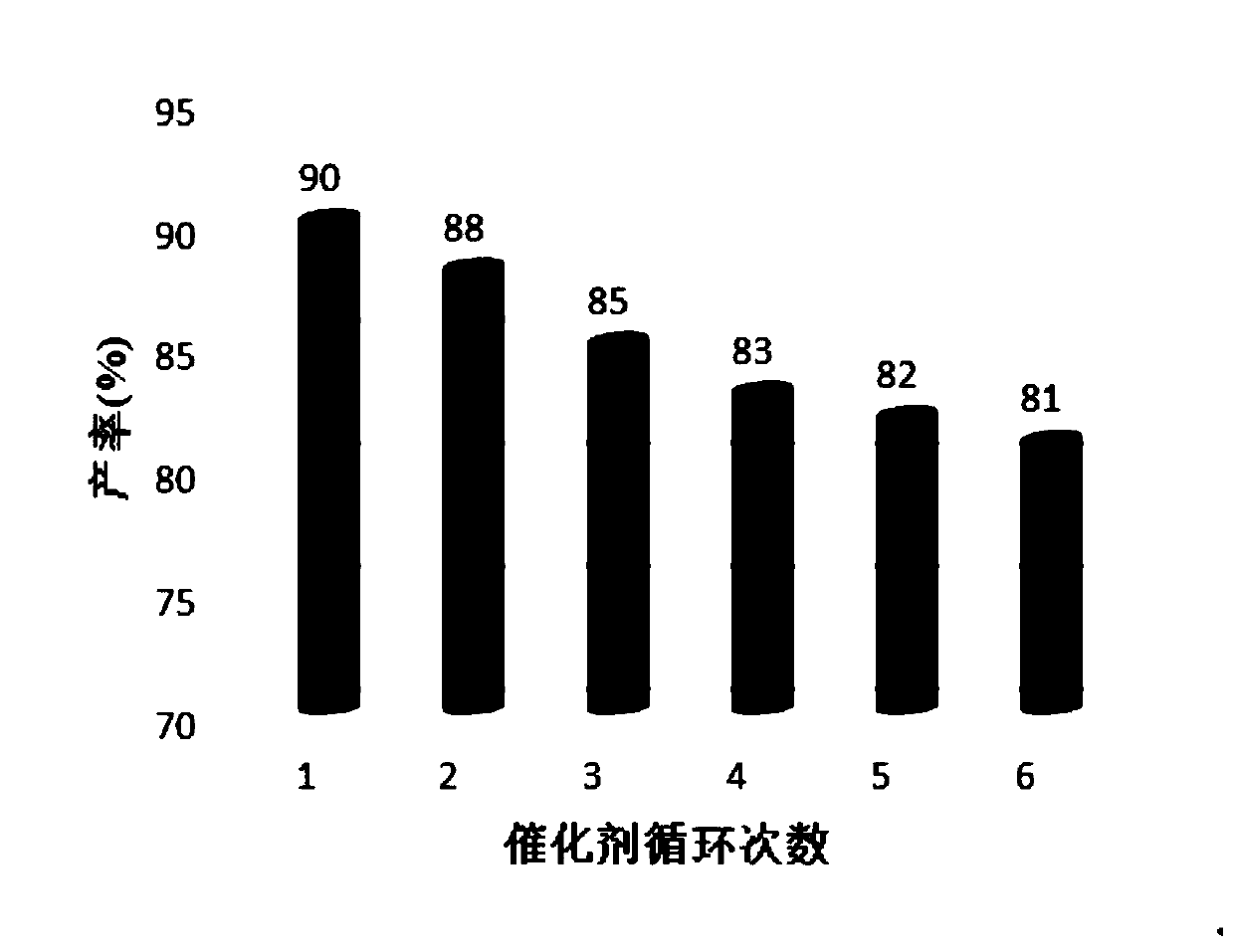
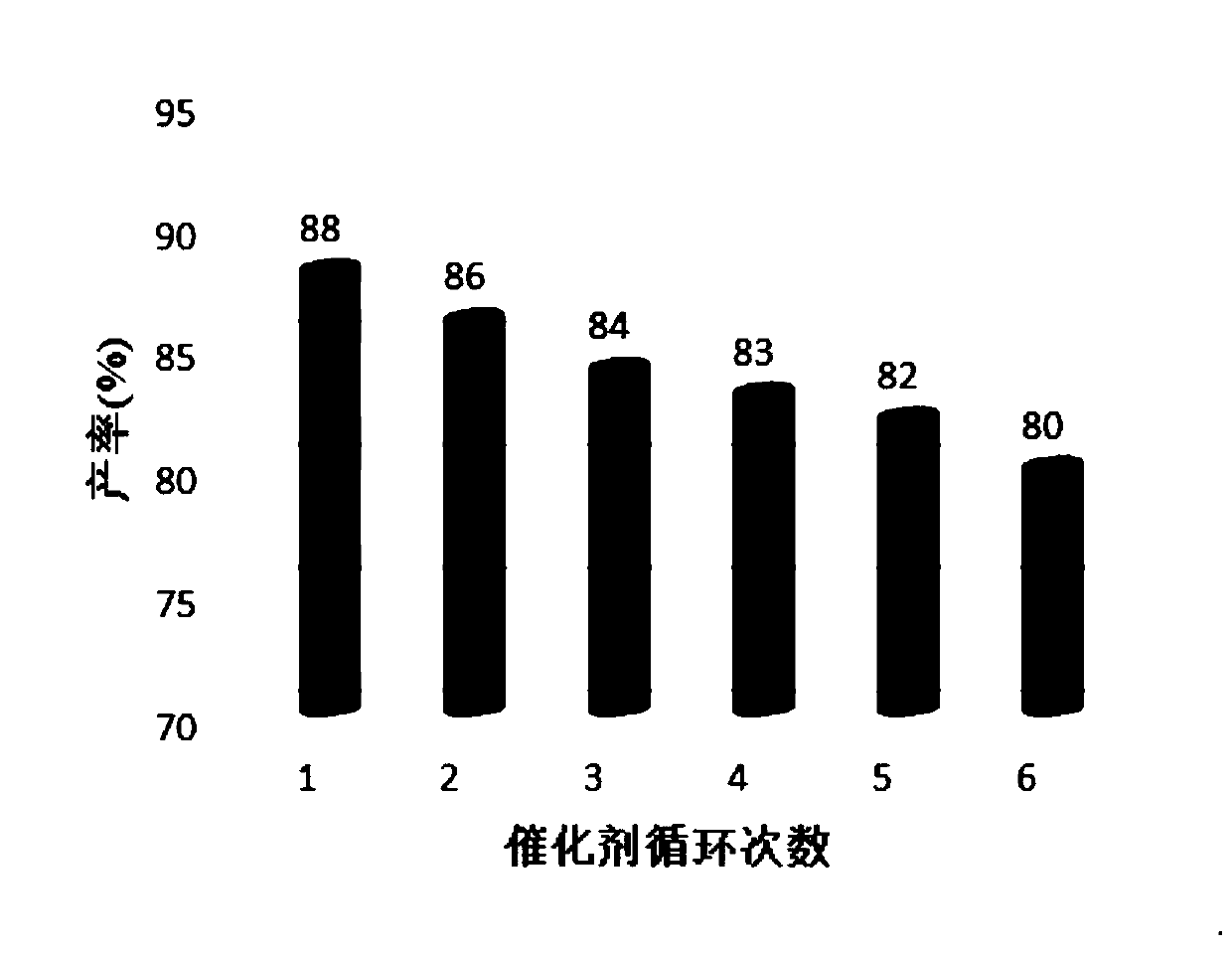
Method for preparing 2,3-dihydroquinazoline-4(1H)-one and derivative thereof
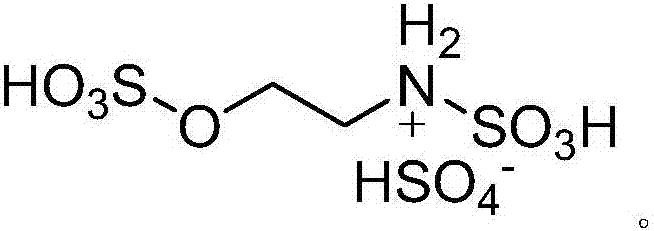
ActiveCN104744380ALow toxicitySimple structureOrganic chemistryChemical recyclingChemical synthesisFiltration
The invention discloses a method for preparing 2,3-dihydroquinazoline-4(1H)-one and a derivative thereof and belongs to the technical field of organic chemical synthesis. In the preparation, the mole ratio of isatoic anhydride to aromatic amine to aromatic aldehyde is 1 to 1 to 1; the molar weight of a high-acidity ionic liquid catalyst is 8% to 12% of that of the used isatoic anhydride; the reflux reaction time is 4min to 30min; the volume dose of a mixed solution of reaction solvents water and ethanol is 3-6 times that of the isatoic anhydride; the reaction pressure is one atmospheric pressure; after the reaction is ended, a product is cooled to room temperature and subjected to suction filtration; the obtained filter residues are dried in vacuum to obtain pure 2,3-dihydroquinazoline-4(1H)-one and the derivative thereof. In comparison with a preparation method adopting other catalysts, the method disclosed by the invention has the characteristics of being relatively small in catalyst toxicity, recyclable in use, relatively good in biodegradability, environmentally friendly and economical during the overall preparation process, simple and convenient to operate, convenient for large-scale industrial production and the like.
Owner:平邑现代中药产业园有限公司
Method for selectively synthesizing polysubstituted dihydroquinazolinone or quinazolinone
ActiveCN110372611AHigh yieldHigh activityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSolvent freeIsatoic anhydride
The invention discloses a method for selectively synthesizing polysubstituted dihydroquinazolinone or quinazolinone. According to the method, heteropolyacid ion liquid is taken as a catalyst, microwave heating and solvent-free synthesis technologies are utilized, and dihydroquinazolinone and quinazolinone derivatives are selectively synthesized through a 'one-pot-method' synthetic strategy with isatoic anhydride or derivatives of the isatoic anhydride, amine and aldehyde as raw materials. Compared with the prior art, the method has the multiple advantages that the efficiency is high, the costis low, environmental friendliness is realized, reaction selectivity is good, the product yield is high, selective synthesis can be realized, the catalyst is convenient to recover and apply, operationis easy, and industrial mass production is convenient, and the method is an environment-friendly efficient selective synthesis novel method and meets the environment-friendly and chemical developmentidea.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
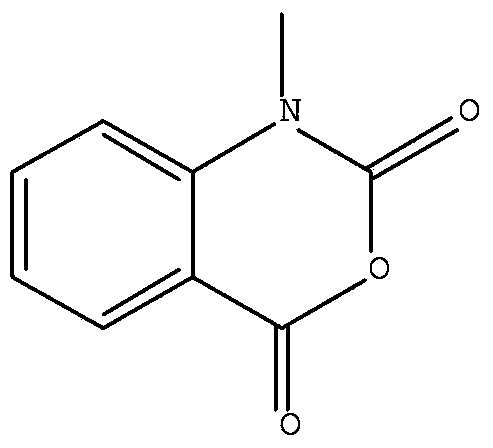
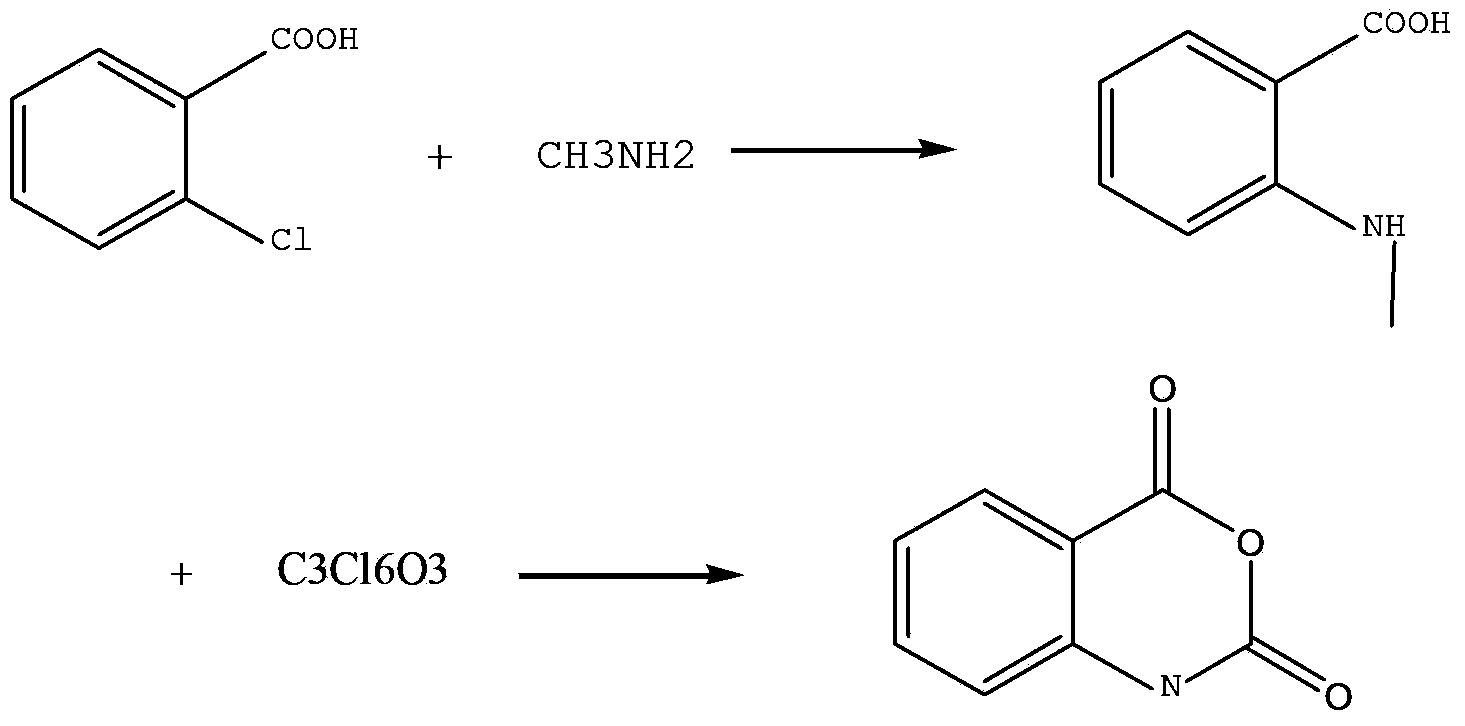
Method for preparing N-methyl isatoic anhydride
ActiveCN103450107AThe synthetic route is simpleSimple and fast operationOrganic chemistryIsatoic anhydrideCopper
The invention provides a method for preparing N-methyl isatoic anhydride. The method comprises simple steps, is simple and convenient to operate, and is suitable for industrial production, and the raw materials are cheap and are easily available. The method comprises following steps of firstly taking anhydrous potassium carbonate as acid-binding agent and copper powder as a catalyst, carrying out a condensation reaction on o-chlorobenzoic acid and methylamine so as to obtain N-methyl o-aminobenzoic acid, and carrying out cyclization through condensation by adopting triphosgene and N-methyl o-aminobenzoic acid, so as to prepare the N-methyl isatoic anhydride.
Owner:SHAANXI JIAHE PHYTOCHEM
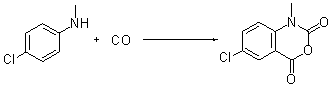
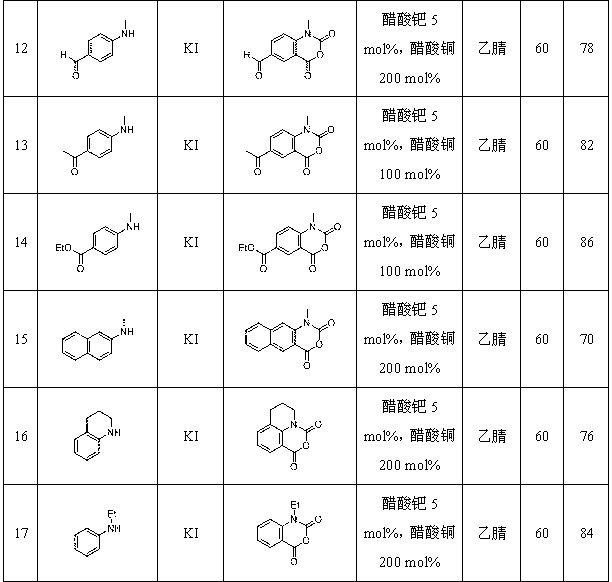
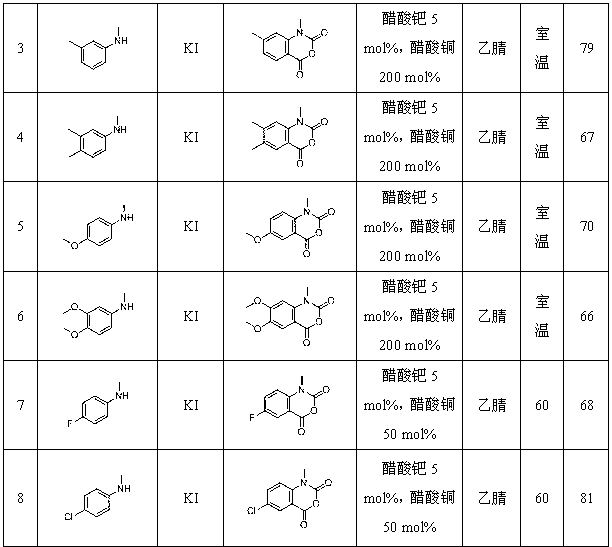
Preparation method of isatoic anhydride derivative
The invention discloses a preparation method of an isatoic anhydride derivative. The preparation method comprises the following steps: in the presence of a palladium catalyst and a copper catalyst, dissolving N,N-dialkyl substituted arylamine and a catalytic amount of an inorganic salt in an organic solvent, and mixing uniformly; and then reacting for 40-55 hours in an atmosphere with a mixed gas of carbon monoxide and oxygen at 60-80 DEG C, and purifying to obtain the isatoic anhydride derivative. The preparation method is simple, the raw material, namely N,N-dialkyl substituted arylamine which is simple and easily available is used for constructing and synthesizing the isatoic anhydride derivative by one step, the preparation condition is mild, and the target product can be highly-selectively obtained in the atmosphere with the mixed gas of carbon monoxide and oxygen at 60 DEG C. The catalytic amount of the inorganic salt is used in the method to create a very good substrate applicable atmosphere, so that the range of a substrate is greatly expanded, the preparation of an isatoic anhydride compound with a practical value is realized, and the preparation method has a very great application potential in the aspect of drug synthetic intermediates and natural products.
Owner:WUHAN UNIV
A kind of preparation 2, the method for 3-dihydroquinazoline-4 (1h)-ketone and derivatives thereof
ActiveCN104744380BSimple structureBiodegradableOrganic chemistryChemical recyclingChemical synthesisFiltration
The invention discloses a method for preparing 2,3-dihydroquinazolin-4(1H)-one and its derivatives, belonging to the technical field of organic chemical synthesis. In the preparation reaction, the molar ratio of isatoic anhydride, aromatic amine and aromatic aldehyde is 1:1:1, the molar amount of high-acidity ionic liquid catalyst is 8-12% of the isatoic anhydride used, and the reflux reaction time is 4-30min , the volume of the reaction solvent water and ethanol mixture is 3 to 6 times the molar weight of isatoic anhydride, and the reaction pressure is one atmospheric pressure. After the reaction is completed, it is cooled to room temperature and filtered with suction, and the obtained filter residue is vacuum-dried to obtain pure 2,3- Dihydroquinazolin-4(1H)-one and its derivatives. Compared with the preparation methods using other catalysts, the present invention has the characteristics of less catalyst toxicity, recyclable use, better biodegradability, green and economical whole preparation process, simple and convenient operation, and convenient industrialized large-scale production.
Owner:平邑现代中药产业园有限公司
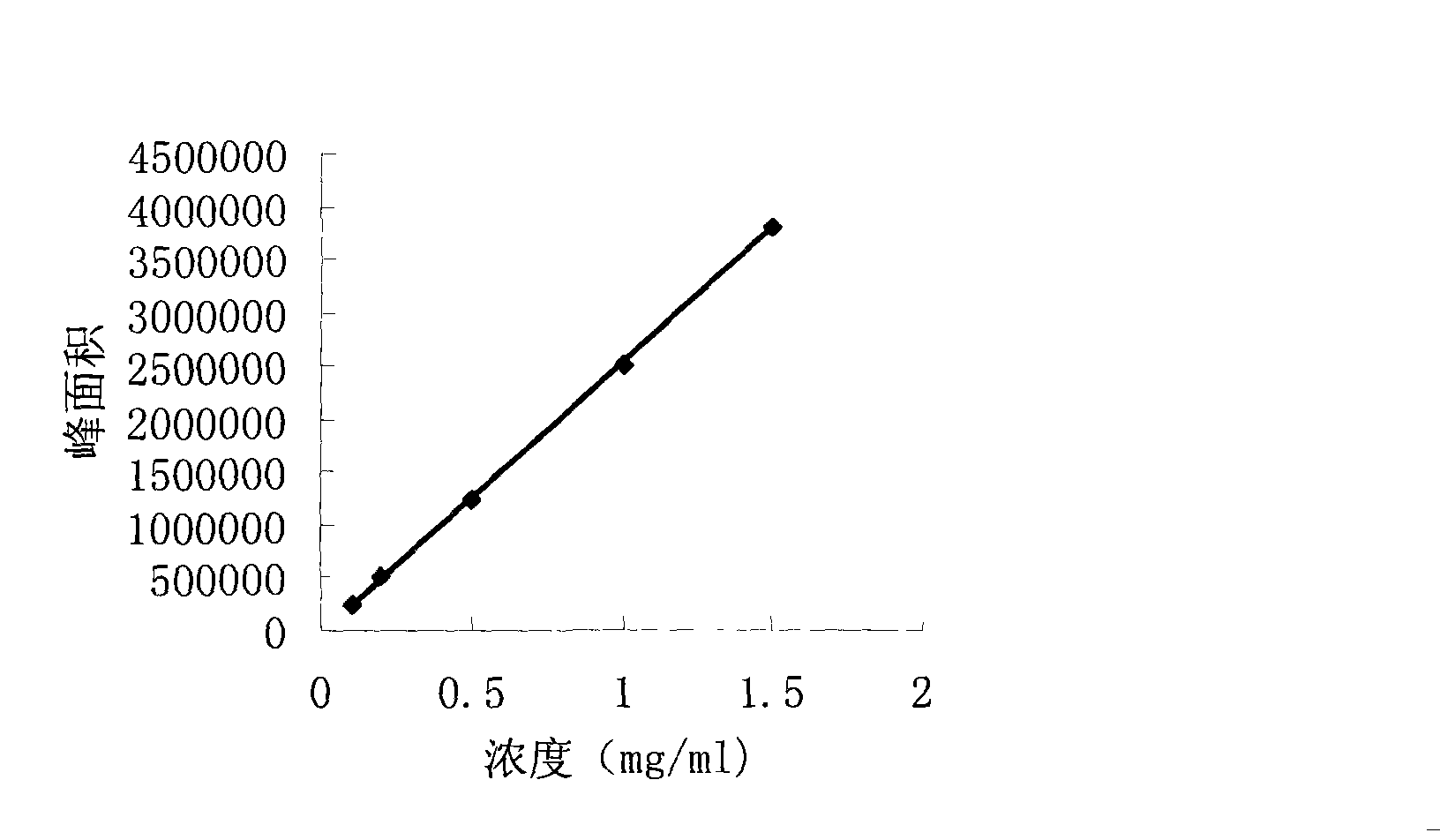
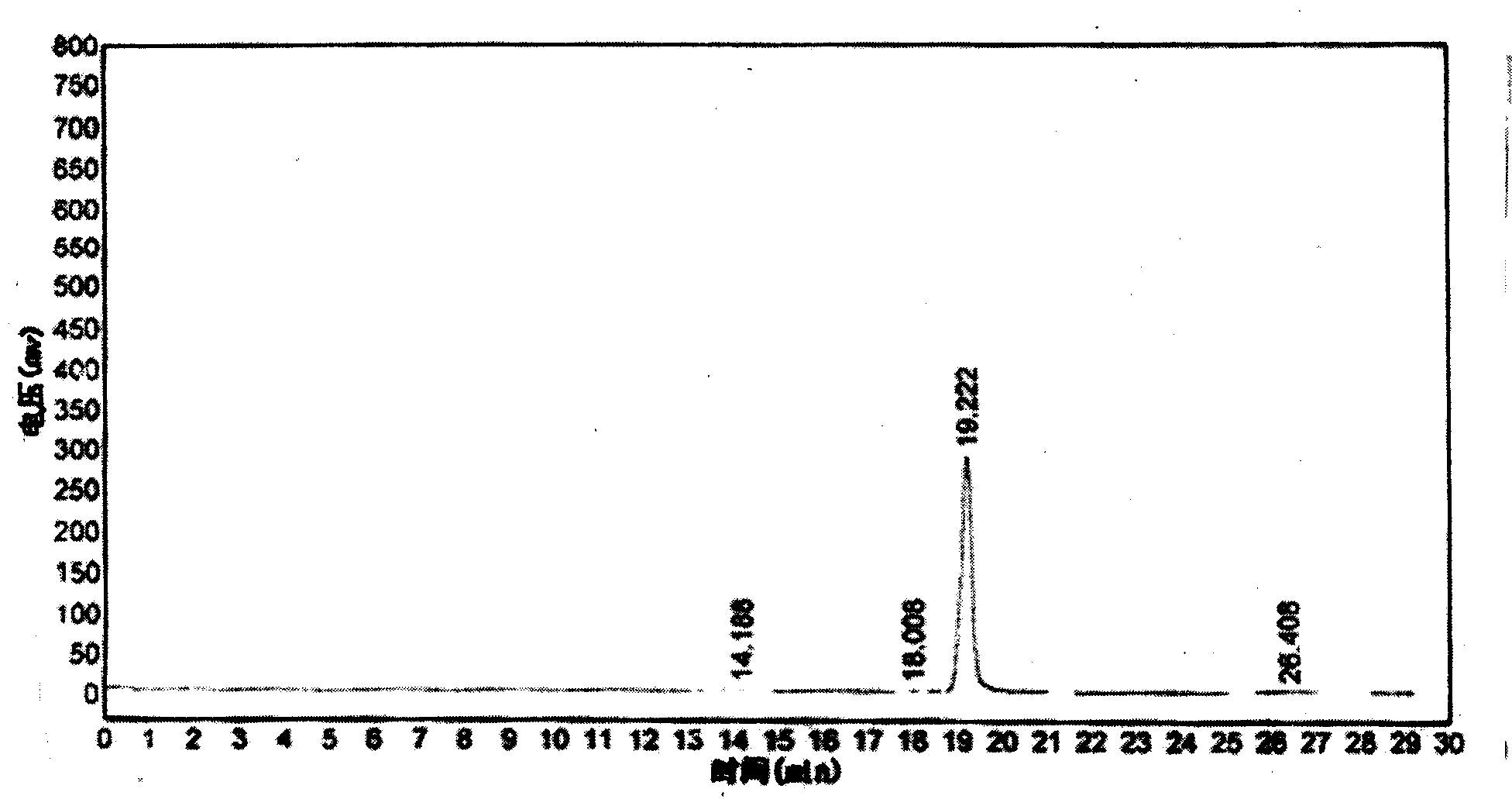
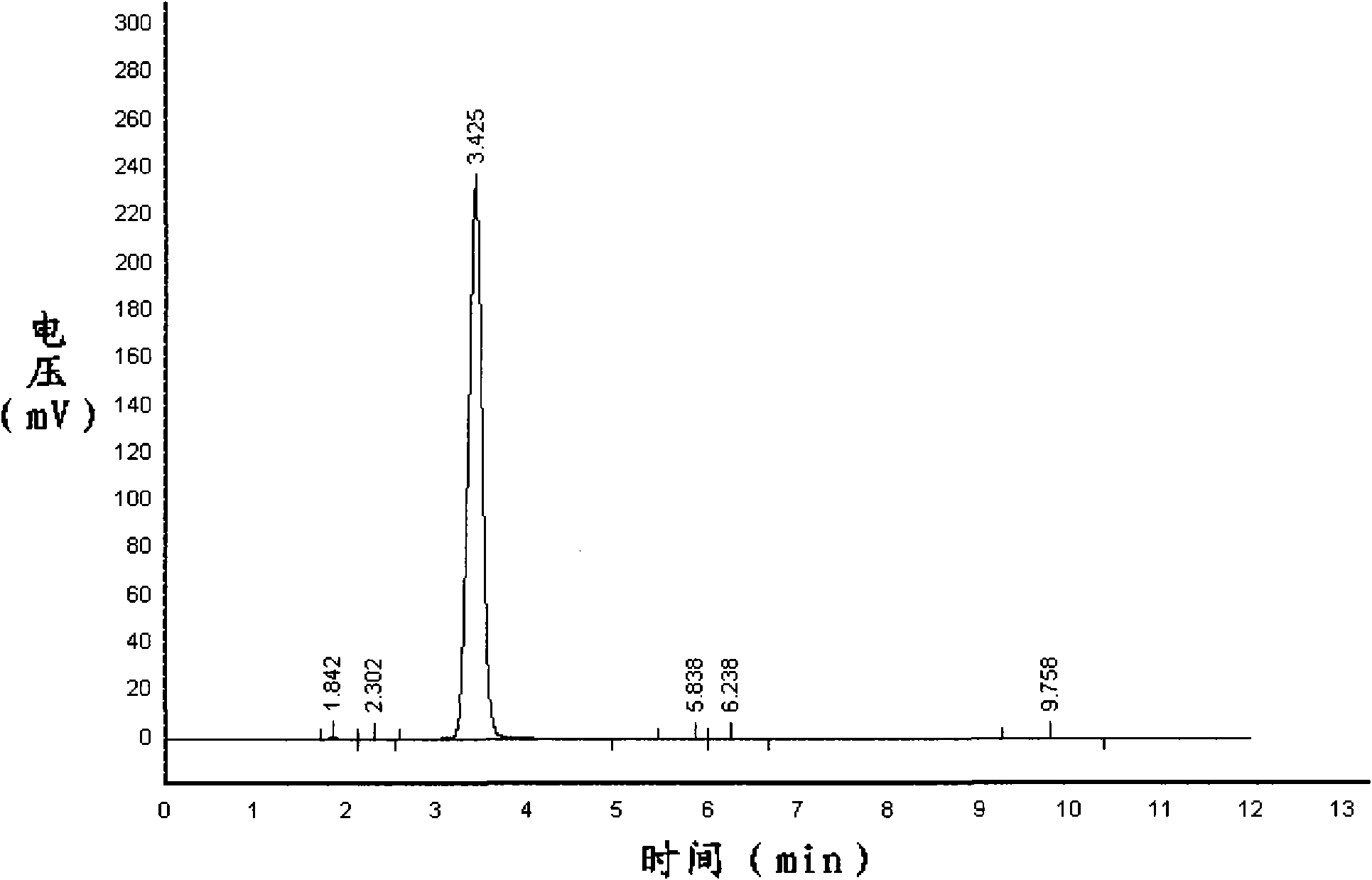
Method for detecting isatin anhydride
InactiveCN101206202AThe detection method is simpleDetection speedComponent separationGas phaseIsatoic anhydride
A detection method for isatoic anhydride belongs to the pesticide technical field, and aims to provide a detection method for isatoic anhydride with simple operation and capable of increasing detection speed and detection accuracy. The method includes the following technical points that: isatoic anhydride sample and standard substance are respectively dissolved by methanol at room temperature condition and then constant volume process is completed; an ODS stainless steel column and an ultraviolet detector are adopted, and the solution of methanol and water is taken as mobile phase with the flow rate controlled at about 0.6ml / min; detection wavelength is adjusted to 254nm, and high efficient liquid phase chromatogram separation and quantification of the isatoic anhydride in the sample is completed. Due to changing the prior indirect gas chromatography to direct liquid chromatography and replacing the gas phase chromatogram detection method adopted after derivation reaction by direct liquid phase detection, the method greatly simplifies the detection method for isatoic anhydride and substantially increases detection speed; meanwhile, because derivation reaction is reduced, the invention reduces detection cost and increases detection accuracy at the same time.
Owner:ANHUI FENGLE AGROCHEM
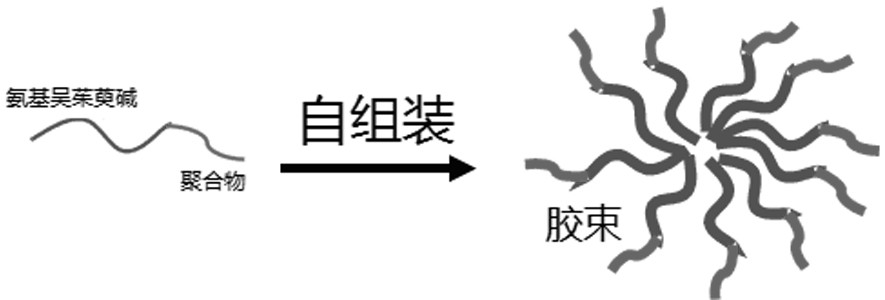
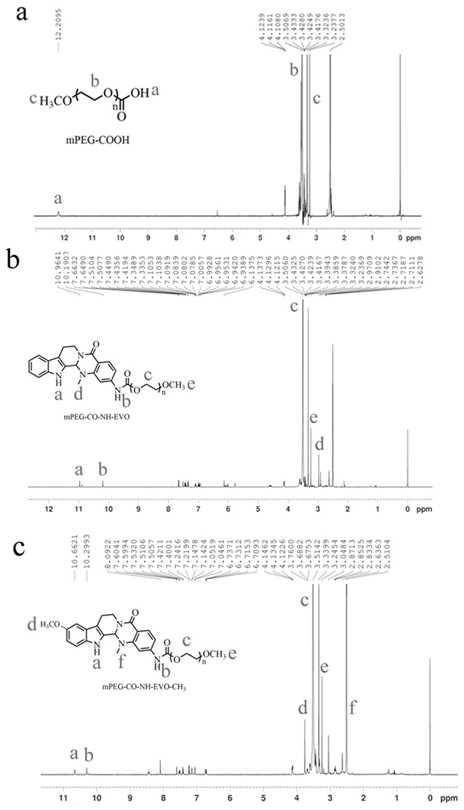
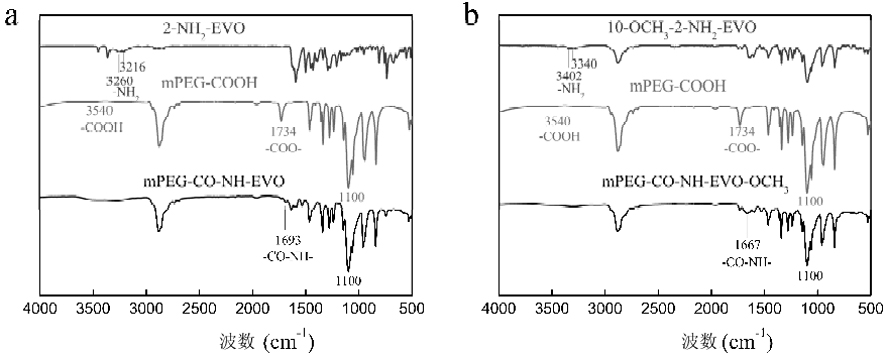
Amino evodiamine polymer micelle as well as preparation method and application thereof
ActiveCN111686077ASignificant induction of apoptosisSignificantly induced proliferative abilityOrganic active ingredientsPharmaceutical non-active ingredientsEvodiaminePolymer science
The invention discloses an amino evodiamine polymer micelle as well as a preparation method and application thereof. The preparation method comprises the following steps: preparing a 2-nitro evodiamine derivative from a carboline compound and N-methyl-7-nitro isatoic anhydride, reducing the 2-nitro evodiamine derivative to obtain an amino evodiamine derivative, and reacting a polymer with amino evodiamine to prepare an amino evodiamine polymer conjugate; and then carrying out self-assembly on the amino evodiamine polymer conjugate to obtain the amino evodiamine polymer micelle. The water solubility and bioavailability of a drug are improved, the drug is combined with a nanotechnology, a nano drug loading system is developed for drug delivery, and the nano drug can be passively enriched intumor tissues in a targeting manner through an EPR effect.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
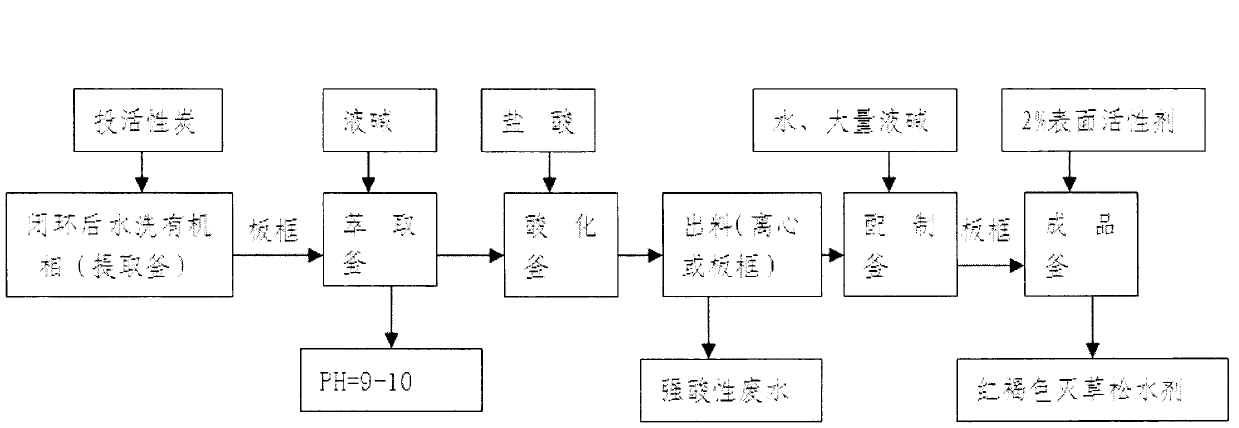
Bentazone decolouring and purifying method
ActiveCN106543101ASolve the problem of deep color, low content and unstable water solutionEasy to operateOrganic chemistryActivated carbonFiltration
The invention relates to a bentazone decolouring and purifying method. The method comprises the following steps: alkali extraction, including taking isatoic anhydride as a raw material, performing synthetic reactions, such as amidation, sulfonation and loop closure, in a dichloroethane solvent system, and carrying out washing and alkali addition for extraction to obtain an alkali-extracted liquid; decolouring, including, transferring the alkali-extracted liquid to a decolouring kettle, adding a certain amount of activated carbon according to the bentazone content, raising the temperature to 40-95 DEG C, and performing heat preservation for 1-5 h; filtration, including after the heat preservation is over, transferring the obtained material to a cooling kettle, performing fast stirring, leading brine in for cooling, performing plate filtration, allowing the obtained filtered liquid to pass an acidification kettle, and then blowing the plate to dry; acidification, including raising the temperature of the filtered liquid in the acidification kettle to 30-70 DEG C, slowly dropwise adding inorganic acid to the filtered liquid till the pH is less than 4, and allowing a great amount of bentazone to precipitate; and drying, including putting the precipitated material in a centrifugal machine for direct spin-drying, and then drying bentazone in a vacuum condition with the temperature in a range of 60-100 DEG C to obtain the final product. Through the method, a white bentazone primary drug is obtained.
Owner:NANJING UNIV
Tryptanthrin derivative and medicinal use thereof
InactiveCN110143964AThe synthesis method is simpleGood at inhibiting tumor cellsOrganic active ingredientsOrganic chemistryCancer cellIsatoic anhydride
The invention discloses a tryptanthrin derivative and a medicinal use thereof. The chemical formula of the tryptanthrin derivative is shown in the description. The tryptanthrin derivative is preparedby a reaction of an isatoic anhydride derivative and an indoquinone derivative. The new compound tryptanthrin with a good inhibition effect on cancer cells is preliminarily screened by MTT experiments. The compound has a pharmacological effect in antitumor.
Owner:NORTHWEST UNIV(CN)
Synthesis method of isatoic anhydride and derivative thereof
ActiveCN103848794AAtom economy is highLess corrosiveOrganic chemistryChemical synthesisSynthesis methods
The invention discloses a synthesis method of isatoic anhydride and a derivative thereof, relating to the technical field of chemical synthesis. In the presence of catalysis of an organic selenium catalyst, isatin or a derivative thereof is oxidized by using hydrogen peroxide to prepare the isatoic anhydride and the derivative thereof. The synthesis method disclosed by the invention is simple and easily available in raw materials, low in cost, clean in oxidizing agent, free from a metal catalyst, and environmentally friendly, can be carried out in a neutral environment, and is small in corrosion to equipment.
Owner:YANGZHOU UNIV
Process for preparing a benzoxazinone
The invention relates to a method for preparing a benzoxazinone comprising reacting an isatoic anhydride with an acylating compound in the presence of an N-alkyl imidazole, to a method for preparing a photo-stabilised composition, comprising adding a benzoxazinone and to the use of a benzoxazinone as a light-absorber or as a stabiliser for a light-sensitive compound.
Owner:TEMSA EURO
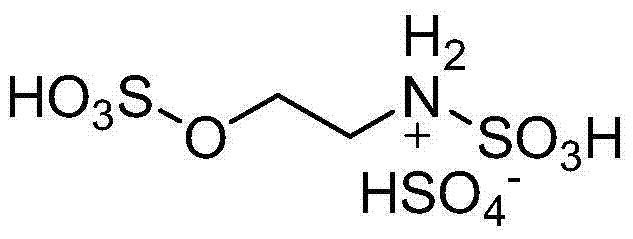
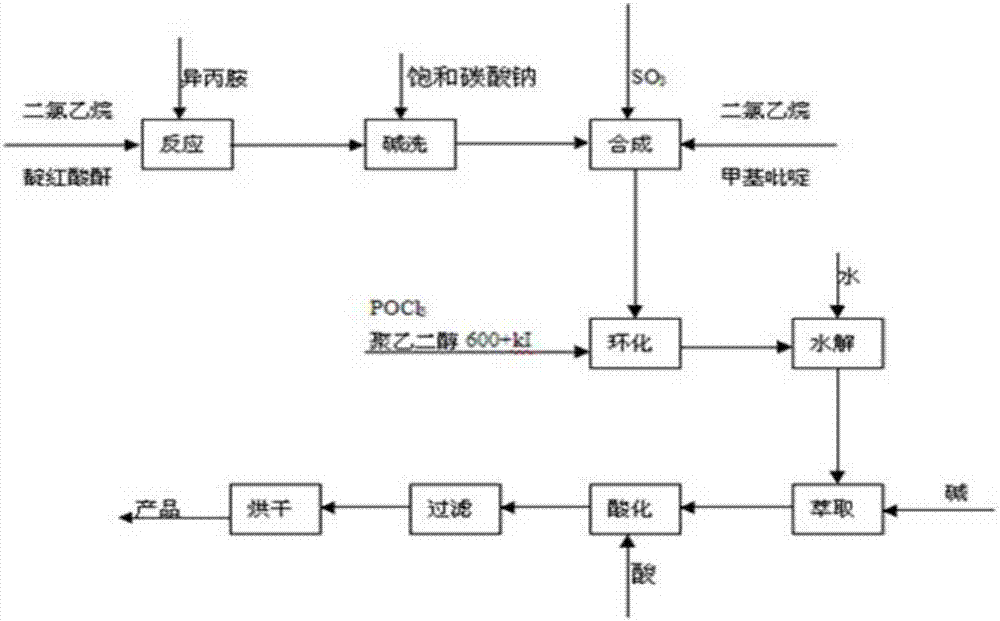
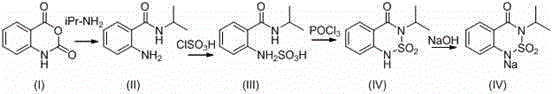
Production method of bentazone technical
The invention discloses a production method of bentazone technical. The production method comprises the following steps: enabling isatoic anhydride, dichloroethane and isopropylamine to react; washing with saturated sodium carbonate to remove the dichloroethane and obtain an o-amino-N-isopropyl benzamide solution; adding alpha-methylpyridine and the dichloroethane into the o-amino-N-isopropyl benzamide solution and mixing; dropwise adding sulfur trioxide, amide dichloroethane, a catalyst and a phosphorus oxychloride solution, and heating till reflux; cooling to room temperature; performing hydrolysis, alkaline washing and water washing; extracting with dilute alkaline liquid; layering to obtain bentazone sodium salt; acidifying and filtering to obtain a light yellow solid; drying to obtain the bentazone technical. According to the production method of the bentazone technical disclosed by the invention, a few steps are adopted, the operation is simple, alkali or acid is not adopted in the production process to ensure little environmental pollution, the production efficiency is high, the yield of the obtained bentazone technical can reach 90% or above, the purity of the bentazone technical is as high as 98.5% or above, and the production method is suitable for industrial production.
Owner:江苏绿叶农化有限公司
Synthetic method for quinazolinone FPR2 formyl peptide receptor agonist
The invention discloses a synthetic method for a quinazolinone FPR2 formyl peptide receptor agonist. The method is specifically implemented according to the following steps of: weighing isatoic anhydride, an aromatic hydrazide compound and an aromatic aldehyde compound with the ratio of amount of substance of 1:1:1 as raw materials; after mixing the raw materials in a solvent, performing a one-pot synthetic reaction by taking a nano metal oxide as a catalyst; and separating and purifying a reaction product after the reaction by virtue of column chromatography to obtain the quinazolinone FPR2 formyl peptide receptor agonist. The synthetic method disclosed by the invention reduces the complexity of preparation by simplifying the preparation process, and moreover, the reaction condition is mild, the raw materials are low in price and are easily available, the product is high in yield, and the solvent in the preparation process is non-toxic, so that the synthetic method has a good industrial application prospect.
Owner:SHAANXI UNIV OF SCI & TECH
Method for preparing bentazone sodium salt powder
The invention discloses a method for preparing bentazone sodium salt powder, mainly relates to a method for preparing a chemical product and mainly aims to provide a method for preparing the bentazone sodium salt powder, which is convenient and practical, saves cost and is suitable for large-scale production. The method comprises the following steps: taking the isatoic anhydride as a raw material, carrying out amide synthesis, sulfonation synthesis and closed-loop synthesis reactions and the like in a dichloroethane solvent system, washing by water, extracting by alkaline water, concentrating, decreasing temperature, crystallizing, drying and packaging to obtain the bentazone sodium salt powder which can be used directly. The main process of the method adopts a simple principle, the repeated steps are omitted, and the cost is saved. The user can use the product directly after the product is packaged, and the method is simple, convenient and environmental-friendly.
Owner:HEFEI XINGYU CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES](https://images-eureka.patsnap.com/patent_img/dd6554d9-cb69-4c3a-84bc-9943330781d5/US20100228023A1-20100909-C00001.png)
![ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES](https://images-eureka.patsnap.com/patent_img/dd6554d9-cb69-4c3a-84bc-9943330781d5/US20100228023A1-20100909-C00002.png)
![ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES ONE STEP PROCESS FOR THE PREPARATION OF SUBSTITUTED 5, 10-DIHYDRODIBENZO [b,e][1, 4]DIAZEPINE-11-ONES](https://images-eureka.patsnap.com/patent_img/dd6554d9-cb69-4c3a-84bc-9943330781d5/US20100228023A1-20100909-C00003.png)