Patents
Literature
46 results about "Tetrakis(triphenylphosphine)palladium(0)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine) is the chemical compound Pd[P(C₆H₅)₃]₄, often abbreviated Pd(PPh₃)₄, or rarely PdP₄. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.
Synthesis method for ortho amino aromatic formic acid aryl ester derivatives
InactiveCN102382001AReduce dosageHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsPalladium catalyst
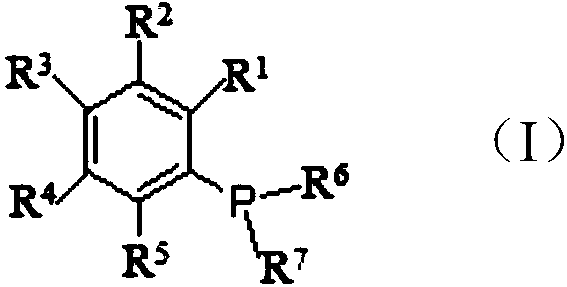
The invention discloses a synthesis method for ortho amino aromatic formic acid aryl ester derivatives as shown in the constitutional formula (I). The method includes steps of utilizing isatoic anhydride compound as shown in the constitutional formula (II) and aryl boric acid as shown in the constitutional formula (III) as raw materials to sufficiently react in inertial organic solvent in the presence of palladium catalyst, phosphine ligands and alkaline compounds, and then obtaining the ortho amino aromatic formic acid aryl ester derivatives by means of separation and purification of reaction liquid after reaction. The palladium catalyst includes one or a combination of the following palladiums: palladium chloride, palladium acetate, tetrakis (triphenylphosphine) palladium, tris (dibenzylideneacetone) dipalladium, dichloro-bis (triphenylphosphine) palladium and dichloro-bis (acetylacetonate) palladium. The synthesis method for the ortho amino aromatic formic acid aryl ester derivatives has the advantages of reasonable technology, low toxicity, mild reaction condition, high reaction yield and fine product quality.
Owner:WENZHOU UNIVERSITY
Preparation method of truxene-based star-like compound
ActiveCN107056828AUniversalThe synthetic route is simpleGroup 3/13 element organic compoundsPorphyrinCycloaddition
The invention discloses a structure and a preparation method of a truxene-based star-like compound (TBP-C60). The preparation method is implemented by the following steps: by taking 7,12-dibromo-truxene-aldehyde derivative as a raw material and taking tetrahydrofuran as a solvent, performing Suzuki reaction on the raw material, the solvent and a BODIPY borate derivative under the catalysis action of tetra(triphenylphosphine) palladium, thus obtaining a truxene-BODIPY binary system compound TB; then by taking the TB and a porphyrin borate derivative as raw materials, performing reaction under the catalysis action of the tetra(triphenylphosphine) palladium, thus obtaining a BODIPY-truxene-porphyrin ternary system compound TBP; then performing cycloaddition reaction on the TBP, sarcosine and fullerene through 1,3-dipole, thus obtaining the star-like compound TBP-C60 in which the truxene is used as a core and the porphyrin, the BODIPY and the fullerene are used as arms. The preparation method of the star-like compound is simple, mild in reaction condition and easy and convenient to operate, shows excellent energy transfer efficiency and high heat stability and can be used for a light absorption antenna, a solar panel, light-simulated biological photosynthesis and other aspects.
Owner:江苏盛叶欣化工新材料有限公司
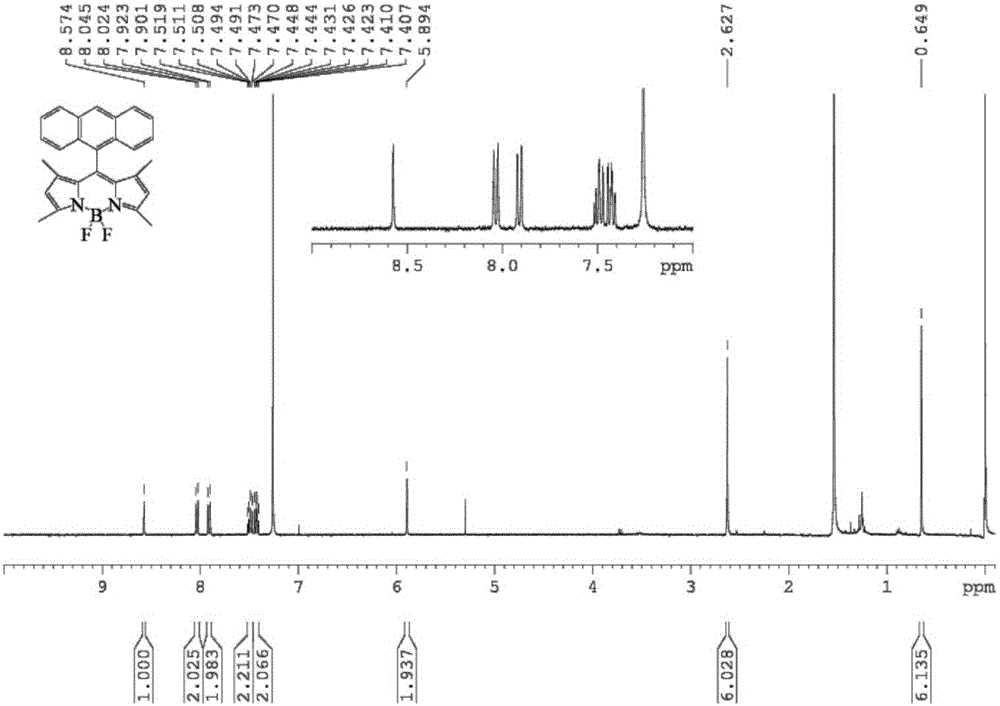
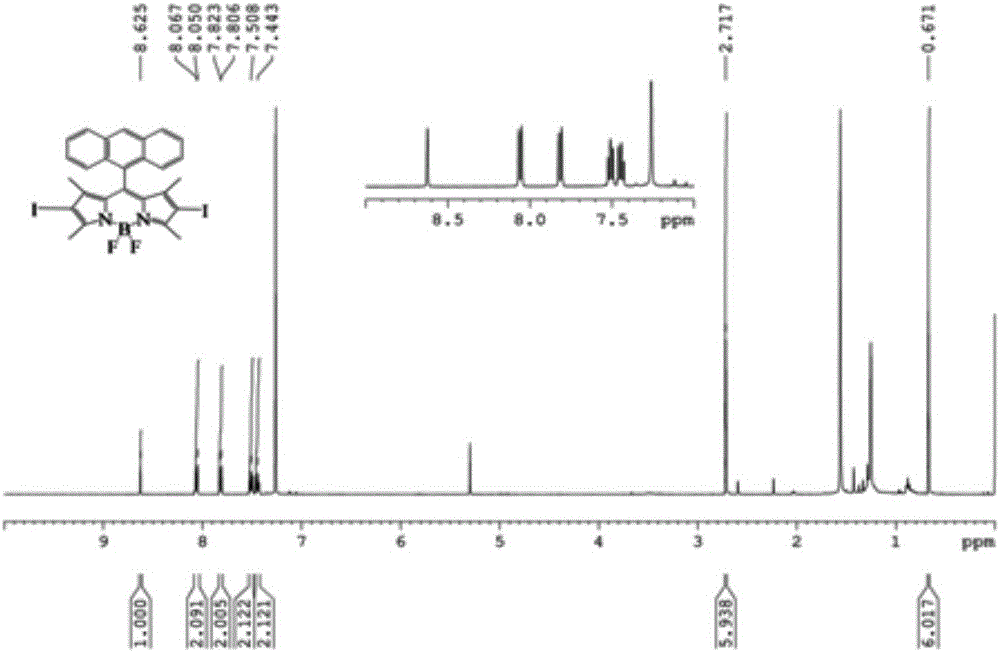
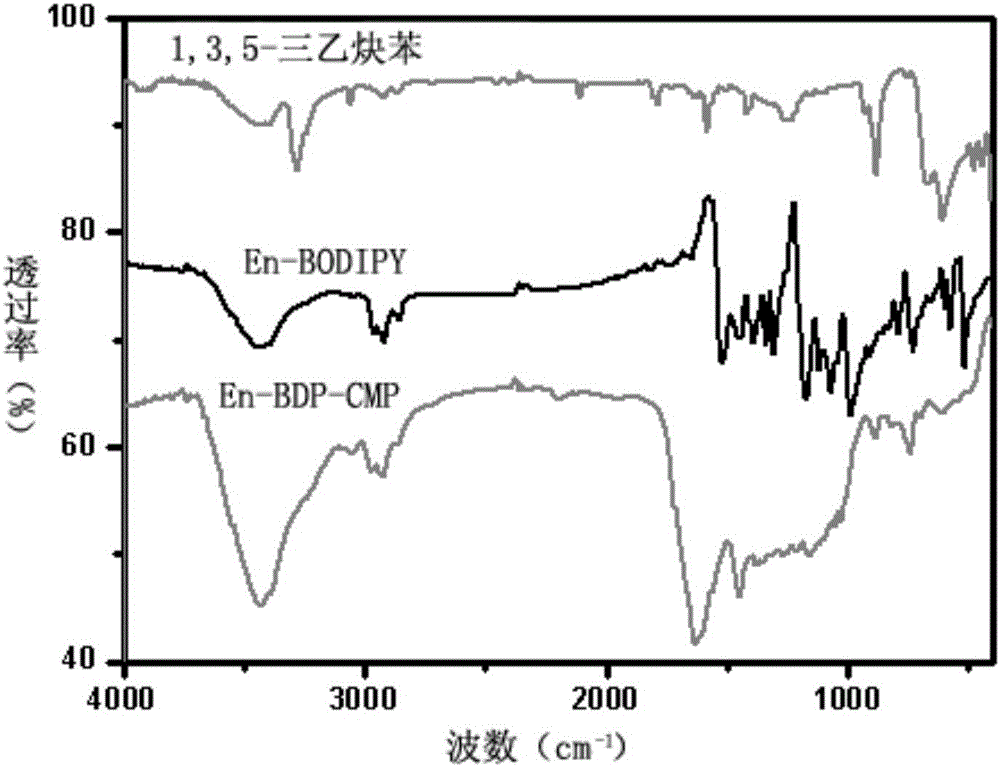
Conjugate microporous polymer based on BODIPY derivative and preparation method thereof
The invention discloses a novel conjugate microporous polymer based on a BODIPY derivative. The conjugate microporous polymer integrally forms a large conjugate skeleton and has high specific surface area, a good porous character, good heat stability and chemical stability and wide application prospects in the fields of gas separation, ion detection, gas absorption, catalysis and the like. The invention further discloses a preparation method of the conjugate microporous polymer. According to the method, 9-anthraldehyde and 2,4-dimethyl-1H-pyrrole serve as raw materials, BODIPY chromophore containing an anthracene group is obtained through multiple steps, then, the BODIPY chromophore containing the anthracene group and 1,3,5-triethynylbenzene are subjected to Sonogashira reaction under catalysis of tetrakis(triphenylphosphine)platinum and CuI, and the conjugate microporous polymer is obtained.
Owner:CENT SOUTH UNIV
Aryl-substituted terpyridyl compounds, and preparation method and application thereof
InactiveCN104030974ALuminousSimple reaction conditionsOrganic chemistrySolid-state devicesRefluxTetrakis(triphenylphosphine)palladium(0)
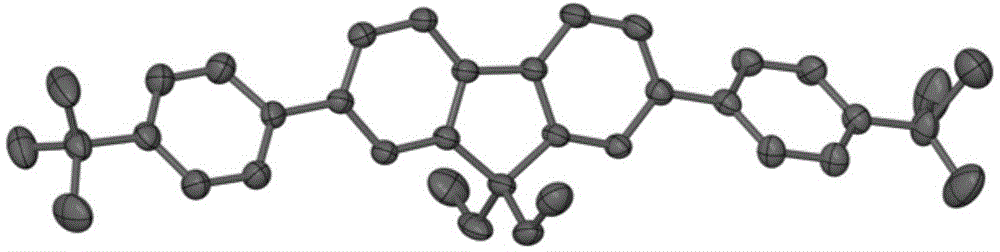
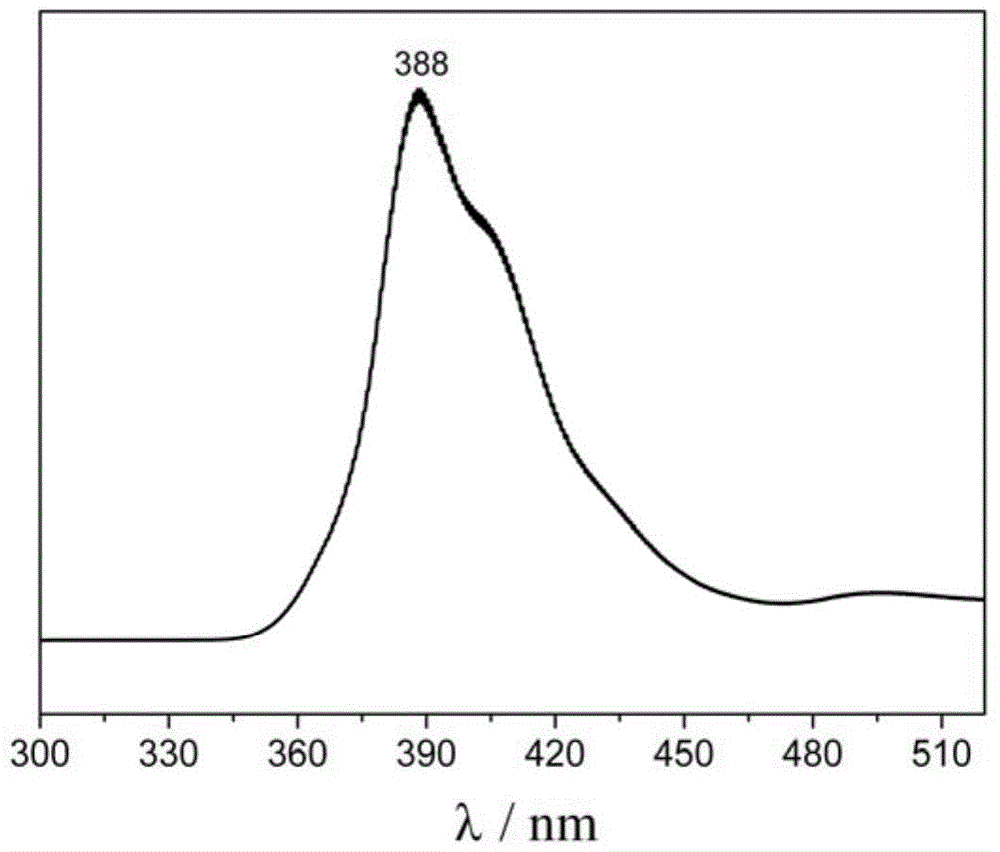
The invention provides aryl-substituted terpyridyl compounds, and a preparation method and application thereof. According to the method, 2-bromophenyl-2,2':6'2''-terpyridyl (II) and aryl borate used as raw materials react under reflux for 18-24 hours in an ultrasonic-degassed mixed solvent system composed of toluene and water in a ratio of 4:1 by using tetra(triphenylphosphine)palladium as a catalyst and potassium carbonate as an alkali. The compounds are suitable for developing and preparing organic luminescent materials and fluorescent materials.
Owner:NANJING UNIV OF TECH
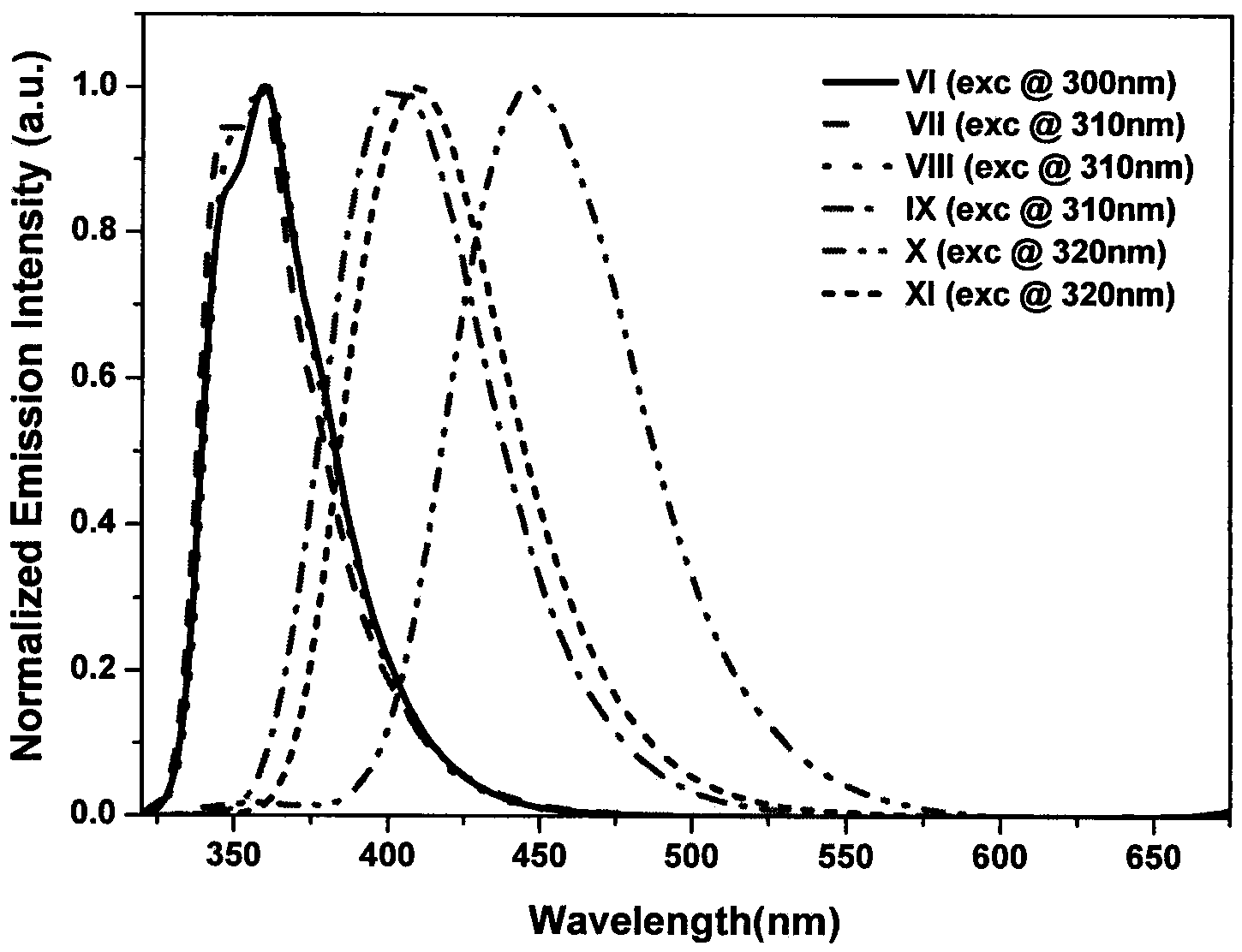
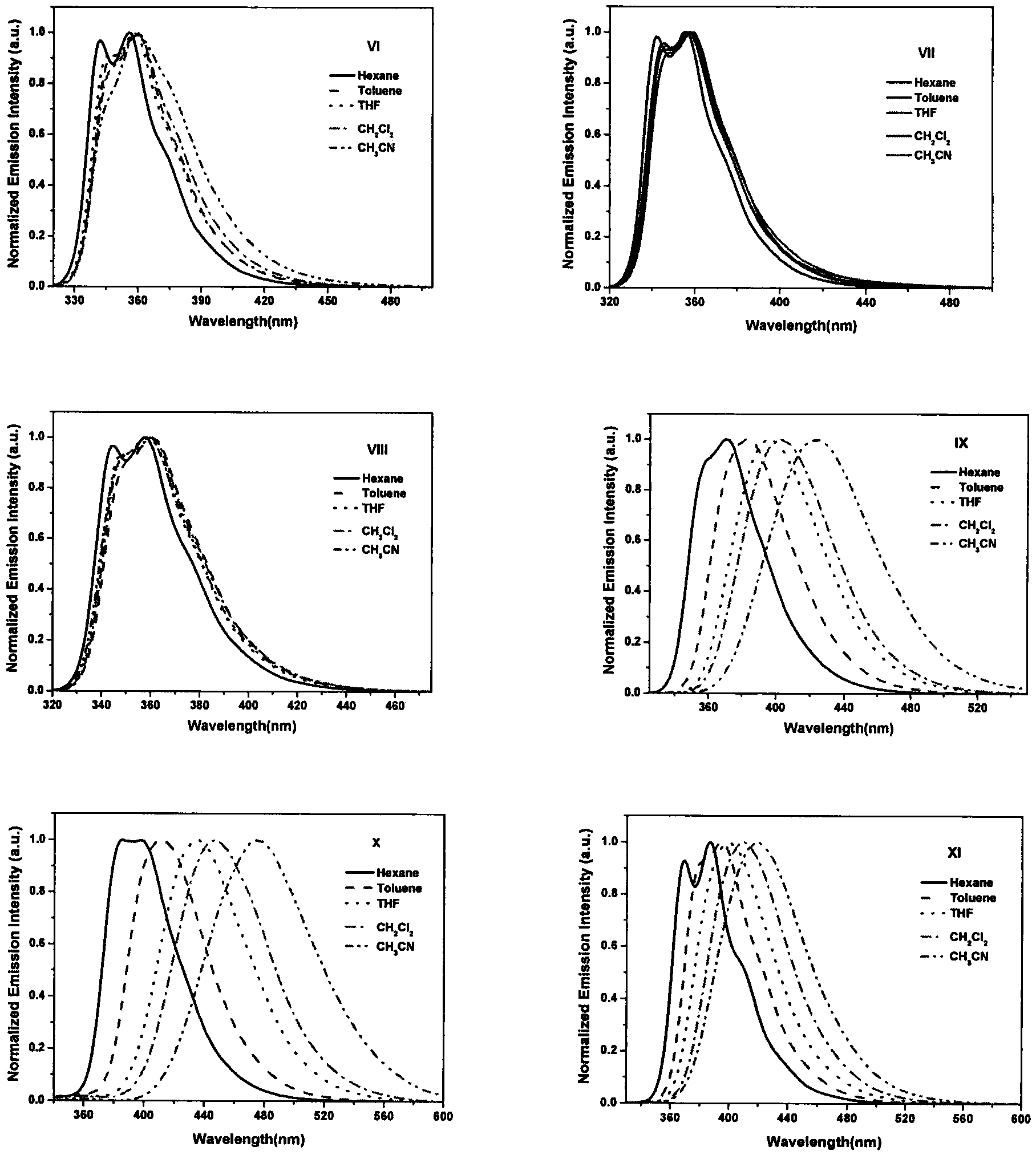
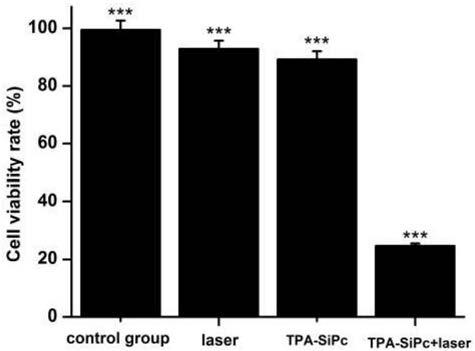
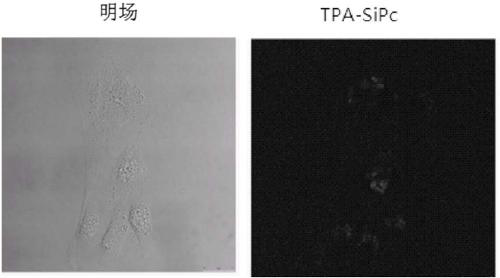
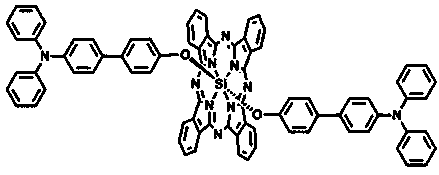
Triphenylamine-based branch ligand substituted silicon phthalocyanine, preparation method and application thereof
ActiveCN108948060AInhibition of self-aggregationImproved Aggregation Fluorescence Quenching PerformanceSilicon organic compoundsEnergy modified materialsTetrakis(triphenylphosphine)palladium(0)Aggregation-induced emission
The invention discloses a triphenylamine-based branch ligand substituted silicon phthalocyanine, a preparation method and an application thereof. 4-bromotriphenylamine and 4-hydroxy phenylboronic acidare catalytically coupled through tetrakis (triphenylphosphine) palladium, so as to acquire a branch precursor 4'-(diphenyl amino)-[1,1'-biphenyl]-4-alcohol; the 4'-(diphenyl amino)-[1,1'-biphenyl]-4-alcohol (TPA-OH) reacts with SiPcCl2 in the presence of methylbenzene and K2CO3, so as to acquire bi-(4-(diphenyl amino)-1-biphenylyloxy) axially substituted silicon phthalocyanine; steric hindranceof a triphenylamine substituted branch structure is capable of restraining the gathering of phthalocyanine to some extent; the triphenylamine with an aggregation-induced emission characteristic is introduced into the branch structure, so that the 'aggregation-induced quenching' effect of phthalocyanine is improved, the optical physical property of phthalocyanine is regulated and the simultaneous execution of fluorescence imaging and photodynamic therapy is realized; the triphenylamine-based branch ligand substituted silicon phthalocyanine can be used as a fluorescence imaging agent and a photodynamic therapy photosensitizer.
Owner:FUJIAN NORMAL UNIV
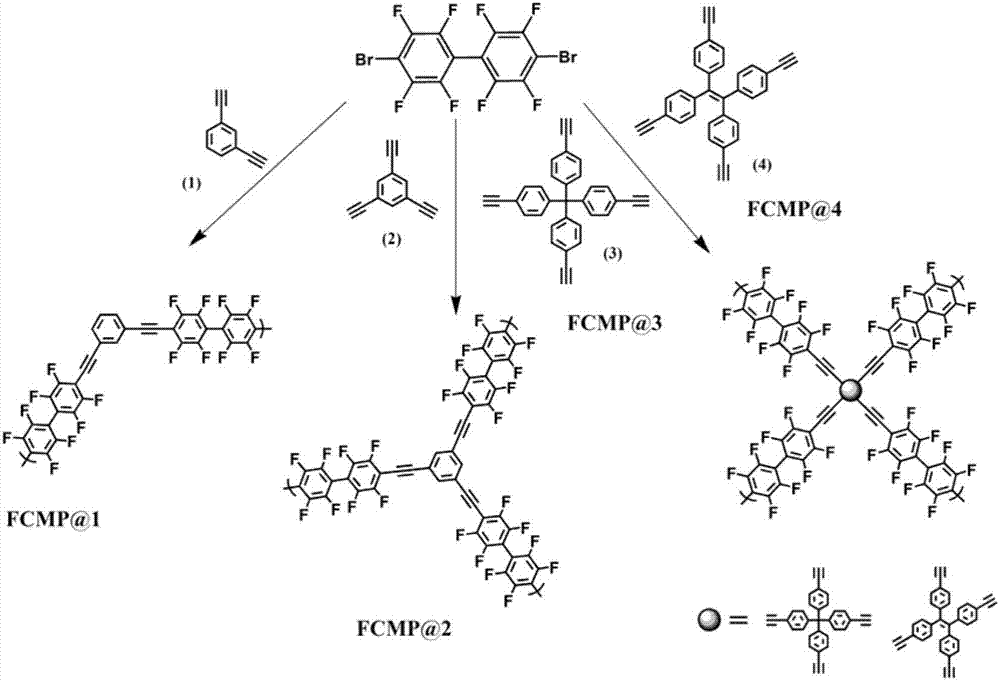
Fluorine-containing porous carbon material as well as preparation method and application thereof
InactiveCN107297196ALarge specific surface areaNarrow pore size distributionGas treatmentOther chemical processesPorous carbonVolumetric Mass Density
The invention discloses a fluorine-containing porous carbon material, which belongs to the technical field of adsorption material preparation. Specifically, the fluorine-containing porous carbon material is prepared in a way that 4,4'-dibromooctafluorobiphenyl and alkyne benzene derivatives are taken as a comonomer, under the catalysis of tetrakis(triphenylphosphine)palladium (0), through Sonogashira, coupling is carried out, and high-temperature calcination is carried out at the temperature of 580-620 DEG C under nitrogen protection. The prepared fluorine-containing porous carbon material has the characteristics of large specific surface area, narrow porous distribution, high physical chemistry stability, low skeleton density and the like, and shows outstanding adsorption capacity for carbon dioxide and iodine.
Owner:JILIN NORMAL UNIV
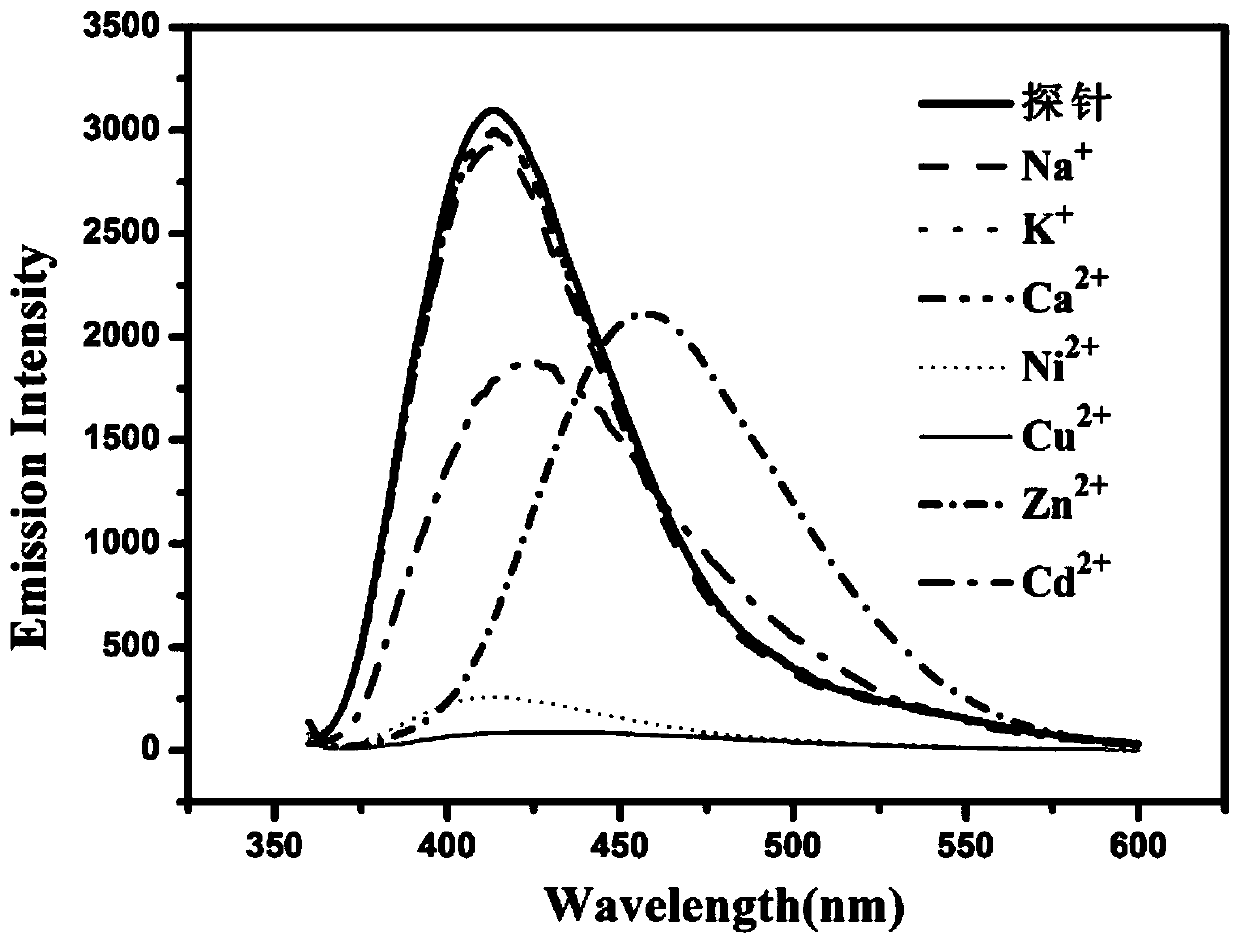
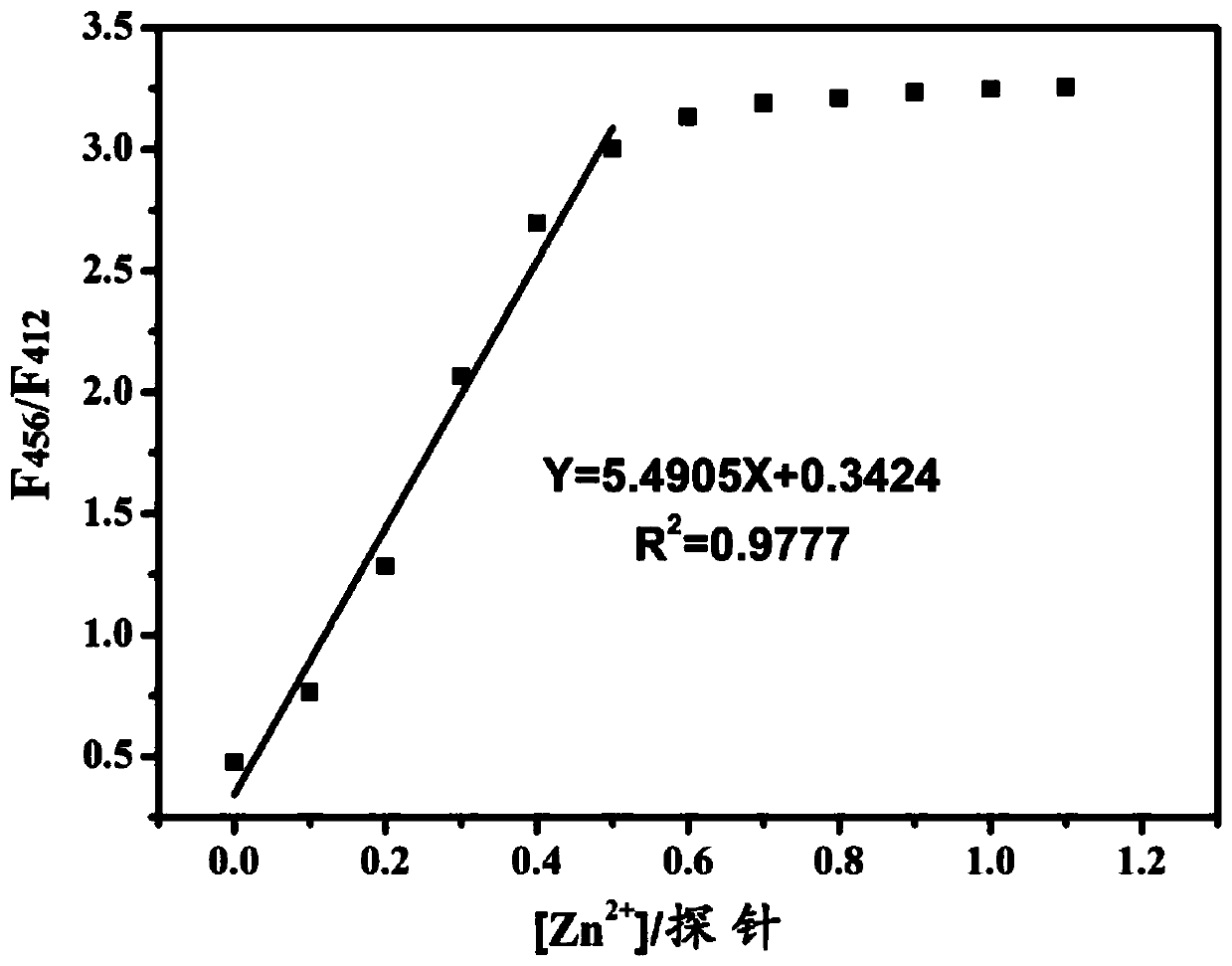
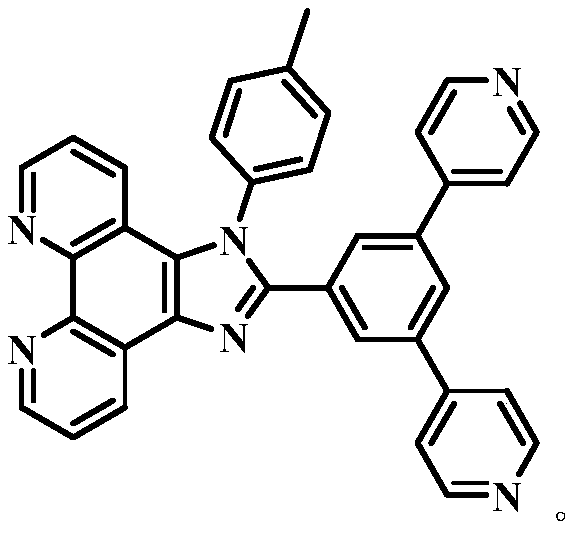
Proportional sensing type zinc ion fluorescence probe and preparing method and application thereof
ActiveCN109776534AQuantitative detection concentrationGood choiceOrganic chemistryFluorescence/phosphorescenceBenzaldehydeFluorescence
The invention belongs to the technical field of ion recognition and particularly relates to a proportional sensing type zinc ion fluorescence probe and a preparing method and application thereof. Thestructure of the probe is shown in the specification. The probe can quantitatively detect the zinc ion concentration and has good selectivity, high sensitivity and low zinc ion detection limit. The preparing method of the probe is also provided and includes adding 3,5-dibromobenzaldehyde, pyridine-4-boronic acid and potassium carbonate into a solvent mixture including toluene, ethanol and water, heating, refluxing and reacting the mixture to obtain 3,5-di(4-pyridinyl) benzaldehyde, with tetrakis(triphenylphosphine) palladium being adopted as a catalyst; then dissolving 1,10-phenanthroline-5,6-dione, the 3,5-di(4-pyridinyl) benzaldehyde, p-toluidine and ammonium acetate into acetic acid, and heating, refluxing and reacting the mixture to obtain 2-(3,5-di(4-pyridinyl)phenyl)-1-p-tolyl-1H-imidazo[4,5-f][1,10]phenanthroline. The synthetic process is simple and easy to achieve. Applications of the probe are also provided.
Owner:SHANDONG UNIV OF TECH
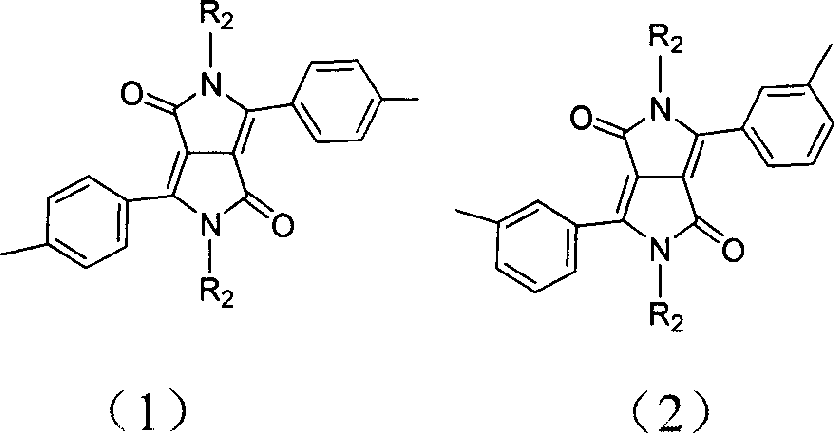
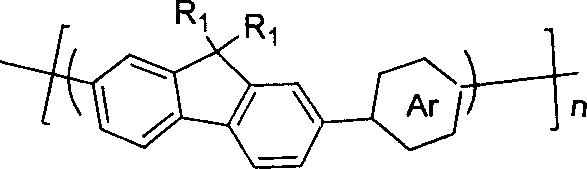
Pyrrolepyrrolidine-diones-fluorene copolymer electroluminescent material and its preparation method
InactiveCN101007943ABroad absorptionAchieve red light emissionElectrical apparatusElectroluminescent light sourcesTetrakis(triphenylphosphine)palladium(0)Organic solvent
The invention discloses pyrrolopyrrolidine-diones-fluorenyl copolymer electroluminescent material and the preparing method. Said electroluminescent material comprises two structural units: pyrrolopyrrolidine-diones and fluorenyl, and the weight average molecular weight is 10000- 100000. The preparing method comprises following steps: dissloving pyrrolopyrrolidine-diones monomer, fluorenes (trimethyleneboric acid ester), tetrapalladium (triphenylphosphine) and K2CO3 in organic solvent under protection of nitrogen gas, reacting at 50- 100 Deg. C; adding 2- bromine fluorenes colsing agent, reacting; cooling, depositing, filtering, washing, extracting and getting final product. The material can adsorb light with wave length being 300- 600 nm, and the maximium electroluminescence wave length is 590- 610 nm, and the light-emitting wave length is in red region.
Owner:SOUTH CHINA UNIV OF TECH
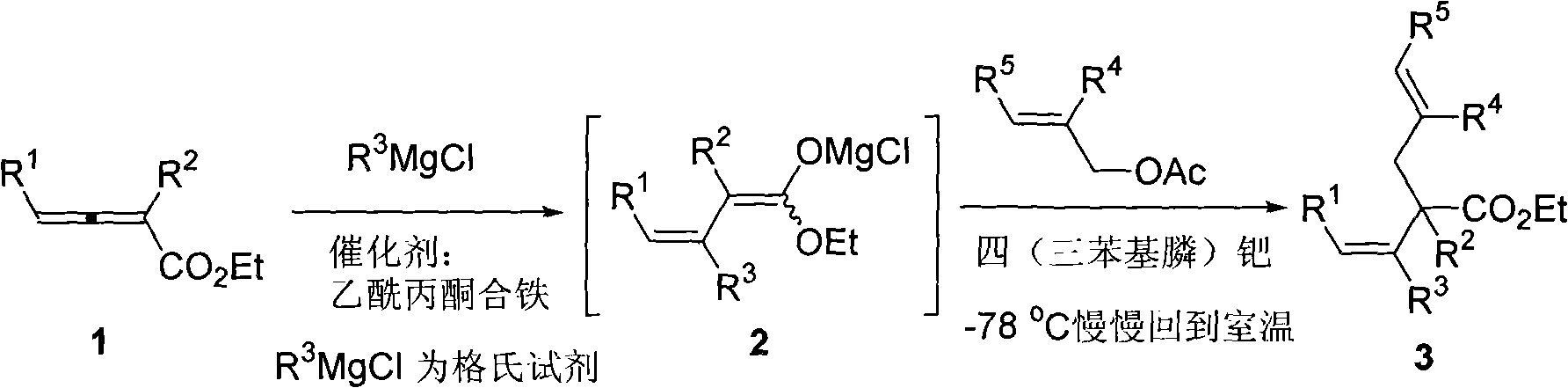
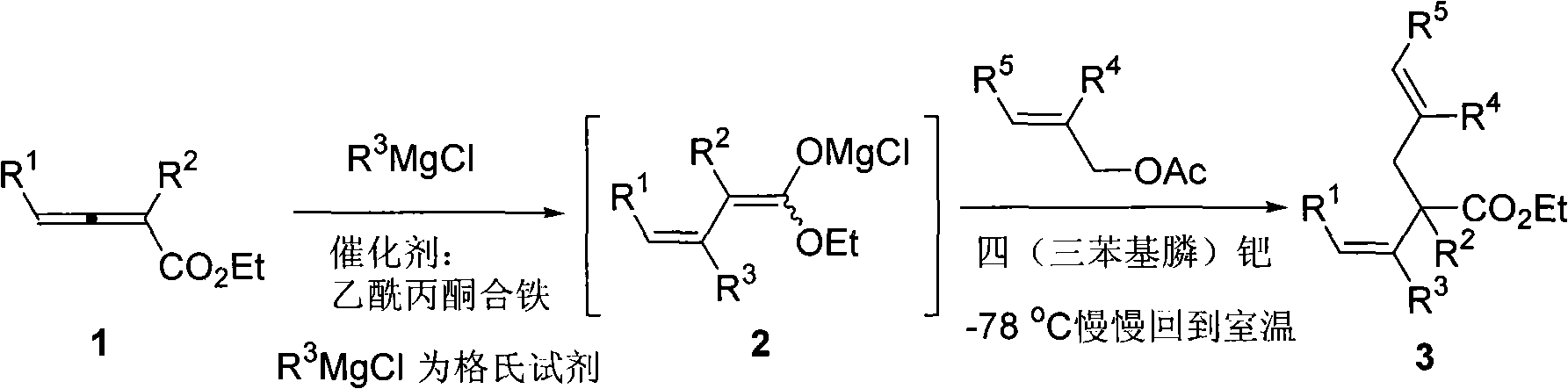
Method for synthesizing cis-alpha-allyl-beta, gamma-unsaturated carboxylic acid ester
InactiveCN101318896AEasy to separate and purifyEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAllyl acetatePlatinum
The invention relates to a cis-alpha-allyl-belta, gamma-unsaturated carboxylic ester and a method for synthesizing the same. The method comprises the following steps that: a1,4-addition of a Grignard reagent and an allenoic ester, is conducted to give a 1,3-conjugated diene magnesia salt at a temperature of below 78 DEG C under the catalytic action of ferric acetylacetonate. The 1,3-conjugated diene magnesia salt reacts with allyl acetates under the catalytic action of tetrakis(triphenylphosphine)platinum to give a series of cis-alpha-allyl-belta, gamma-unsaturated carboxylic ester compounds. The synthetic method has the advantages that the operation is simple, the raw materials and reagents are readily available, the reaction has high regional and vertical selectivity and can substitute two groups at the same time, the products can be separated and purified easily, and can be used to synthesize various cis-alpha-allyl-belta, gamma-unsaturated carboxylic esters.
Owner:ZHEJIANG UNIV
Anticancer active molecular skeleton 1,4-enyne compound, and preparation method and application thereof
ActiveCN109704926AReduce dosageGrowth inhibitionSilicon organic compoundsOrganic compound preparationArgon atmospherePalladium catalyst
The invention discloses an anticancer active molecular skeleton 1,4-enyne compound, and a preparation method and application thereof. The preparation method comprises the following steps: an allyl alcohol raw material, terminal alkyne, tetrakis (triphenylphosphine)palladium, calcium bis(trifluoromethyl sulfonyl)imide and an additive are added into a reaction solvent in sequence; a catalytic reaction is carried out for 12-48 hours at an argon atmosphere at a temperature of 100 DEG C and under a stirring state; a reaction solvent in the reaction solution is removed; and purifying is carried outto obtain the anticancer active molecular skeleton 1,4-enyne compound. The 1,4-enyne compound can be used for medicines inhibiting human esophageal carcinoma cells. The usage amount of a palladium catalyst in the method is 1%, the usage amount of a calcium catalyst is 5%, the usage amounts are extremely small, but an expected effect can be achieved. According to the method disclosed by the invention, the substrate application range is wide, and the allyl alcohol can contain various substituted phenyls, heterocyclic rings and alkyls. The method disclosed by the invention is suitable for different types of allyl alcohol, and the 1,4-enyne compound can be synthesized in a scale of 10 g. The 1,4-enyne compound disclosed by the invention has relatively good anti-cancer activity.
Owner:NANJING UNIV OF TECH
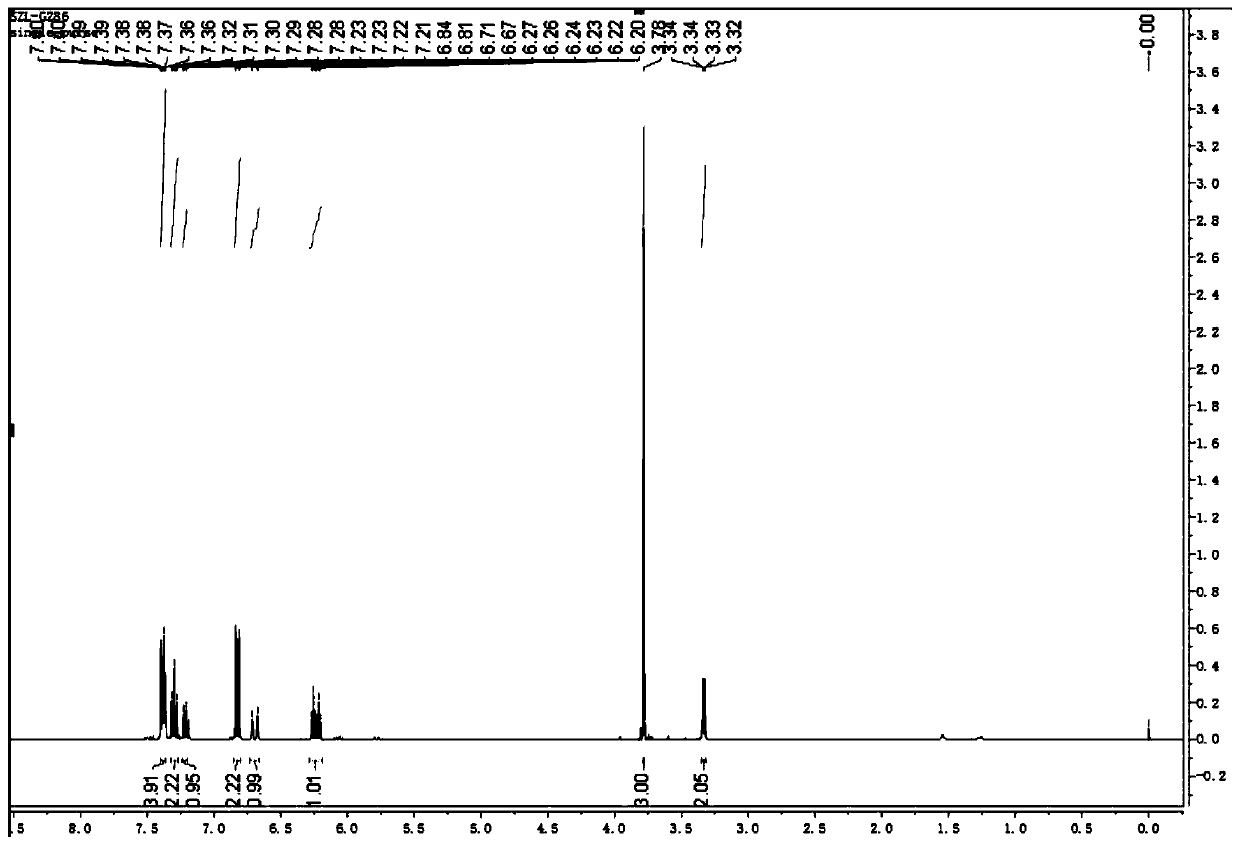
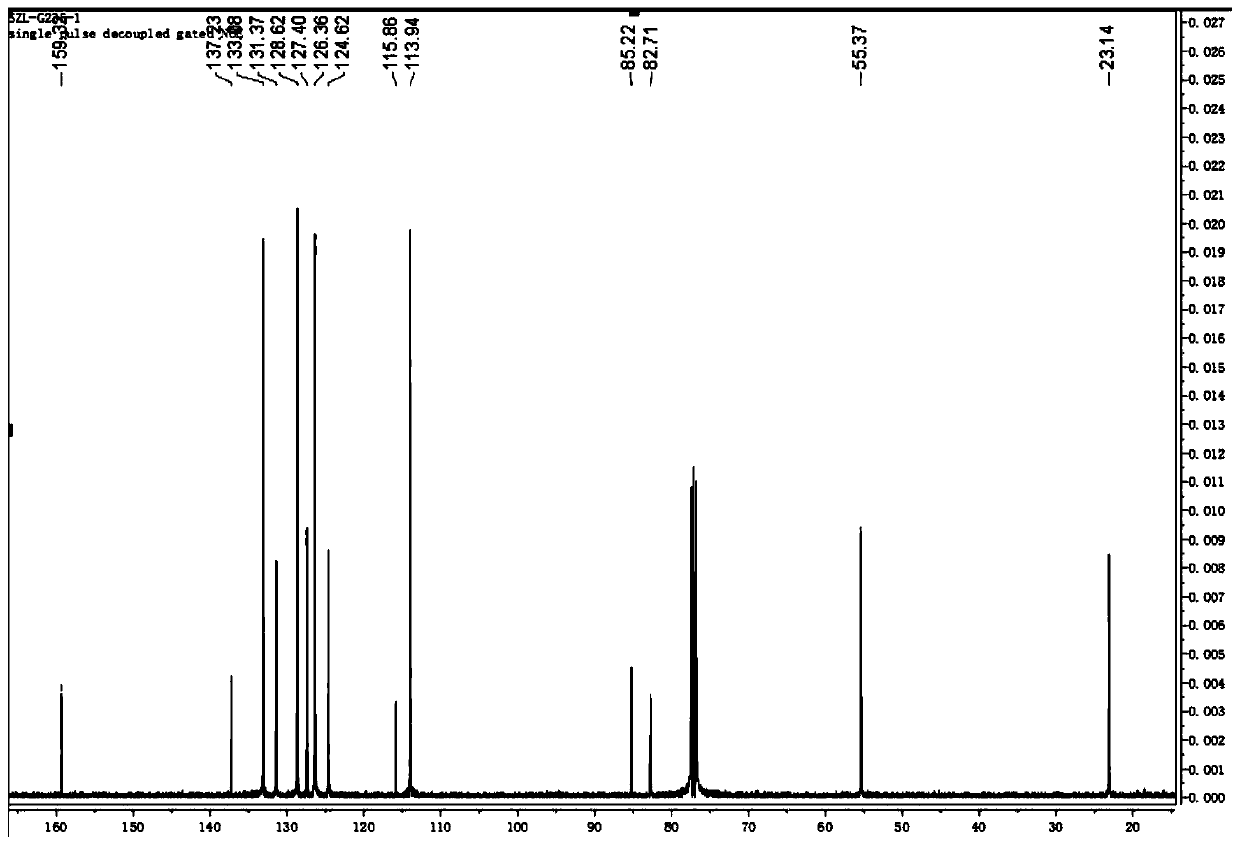
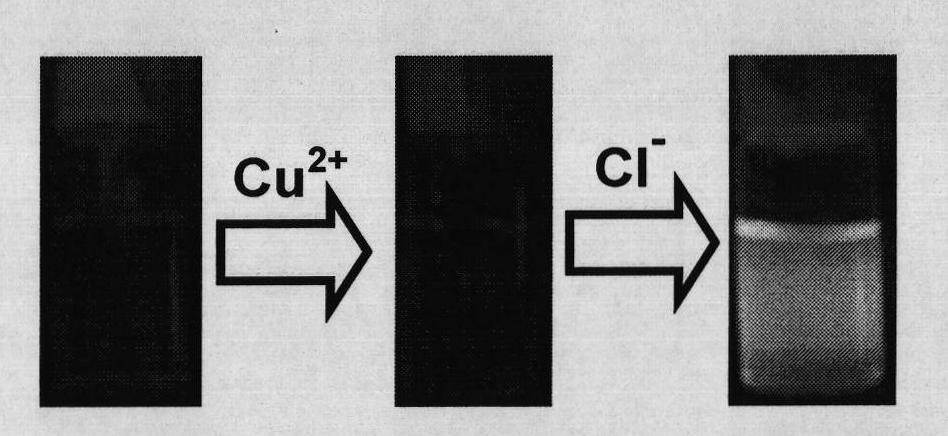
Chloride ion fluorescent probe and preparation method and application thereof
ActiveCN101906299AGood choiceLow detection limitHydrocarbonsFluorescence/phosphorescenceFluorescenceOrganic layer
The invention discloses a chloride ion fluorescent probe and a preparation method and application thereof. The probe comprises 2,7-bis(biphenyl-2-yl)-9-cycloheptatrienylidene and 2,7-bis(biphenyl-4-yl)-9-cycloheptatrienylidene, and is prepared by the following steps of: adding 1 equivalent unit of 2,7-dibromo-9-cycloheptatrienylidene, 2.2 to 3.0 equivalent units of aryl boric acid and 14 equivalent units of potassium carbonate under the protection of nitrogen gas; adding a mixed solvent of methyl benzene, ethanol and water in a volume ratio of 1:1:1 to 2:2:1 into the mixture; adding a catalytic amount of tetra(triphenylphosphine)palladium into the mixture; heating and refluxing the mixture; reacting the mixture for 6 to 12 hours; cooling reaction liquid; extracting the reaction liquid with methylene dichloride; combining organic layers; washing the combined organic layer with saturated saline solution; drying an organic phase through magnesium sulfate; filtering the organic phase; evaporating the solvent; and performing separation by column chromatography to obtain chloride ion fluorescent probe molecules. The probe has the advantages of high ion selectivity, low detection lower limit, high flexibility, simple operation, broad application prospect, high practical value and capability of effectively distinguishing chloride ions from other oxyacid anions and the like.
Owner:ZHEJIANG UNIV
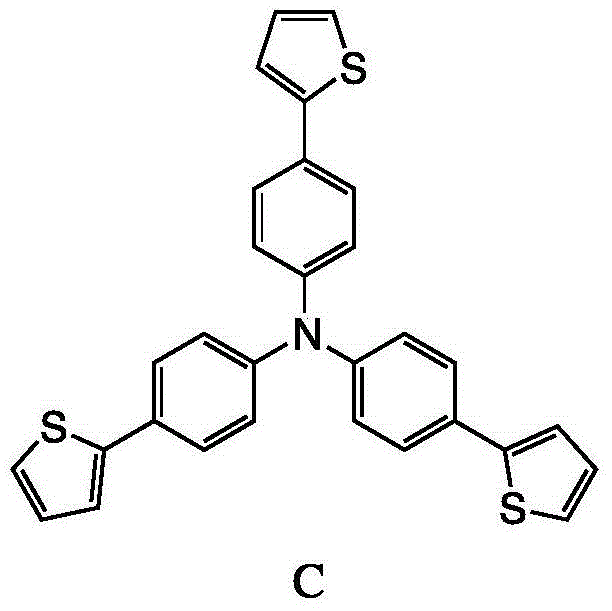
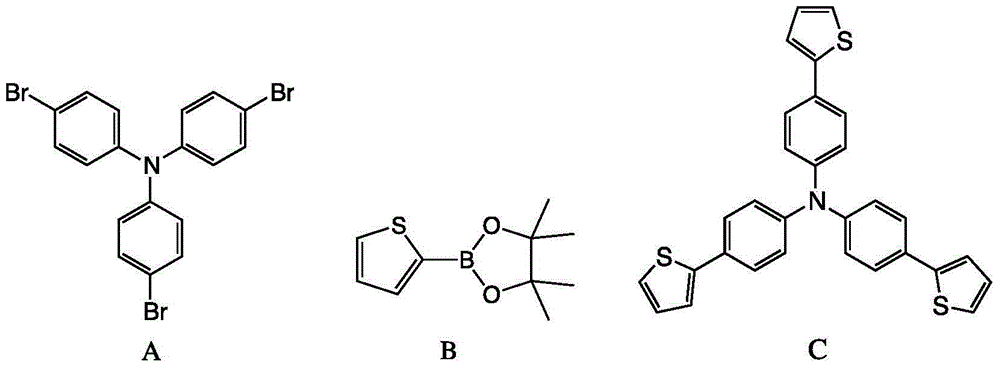
Method for preparing triphenylamine derivative
InactiveCN104592193AReduce usageImprove reaction efficiencyOrganic chemistryTetrakis(triphenylphosphine)palladium(0)Organic solvent
The invention discloses a method for preparing 4,4',4''-tri(2-thienyl)triphenylamine shown as a formula C. The method comprises the following steps: adding a compound shown as a formula A, a compound shown as a formula B, potassium carbonate and tetrakis(triphenylphosphine) palladium into an organic solvent, heating and carrying out a reflux reaction for 30-40 hours under nitrogen protection, thereby obtaining the compound shown as the formula C. According to the system, use of extremely toxic substances such as chloroform is avoided, and dichloromethane and water are used; the reaction efficiency is improved, and the reaction time is greatly reduced; in the column chromatography separation and purification operations, only dichloromethane is used, the process is optimized, and the separation efficiency is improved. The structural formulae are as shown in the specification.
Owner:ZHEJIANG UNIV OF TECH
Blue fluorescent material and preparation method thereof
InactiveCN107739607AImprove thermal stabilityReduce non-radiative inactivationCarboxylic acid nitrile preparationOrganic compound preparationTetrakis(triphenylphosphine)palladium(0)Chemical reaction
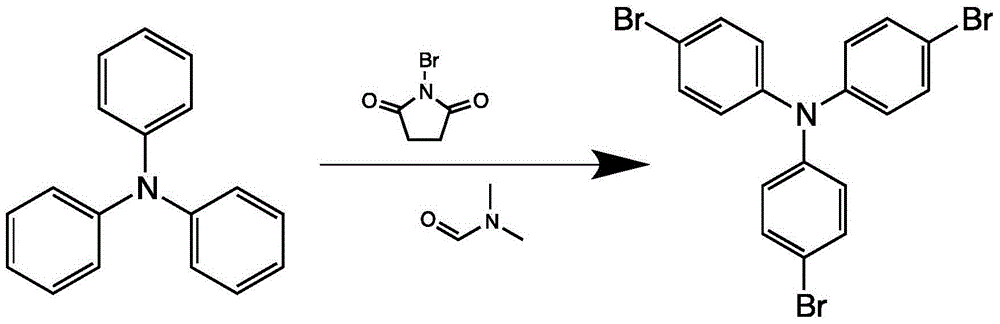
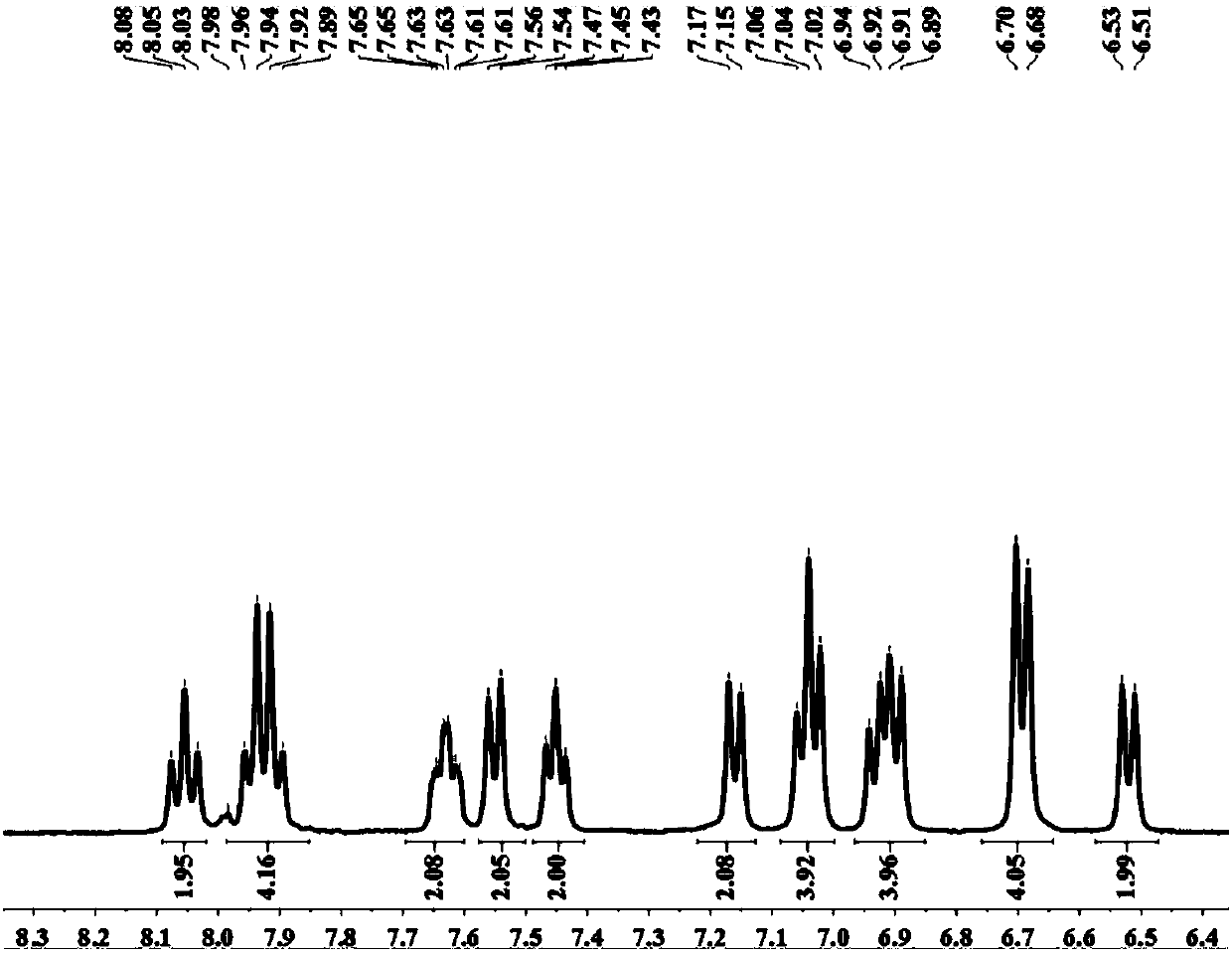
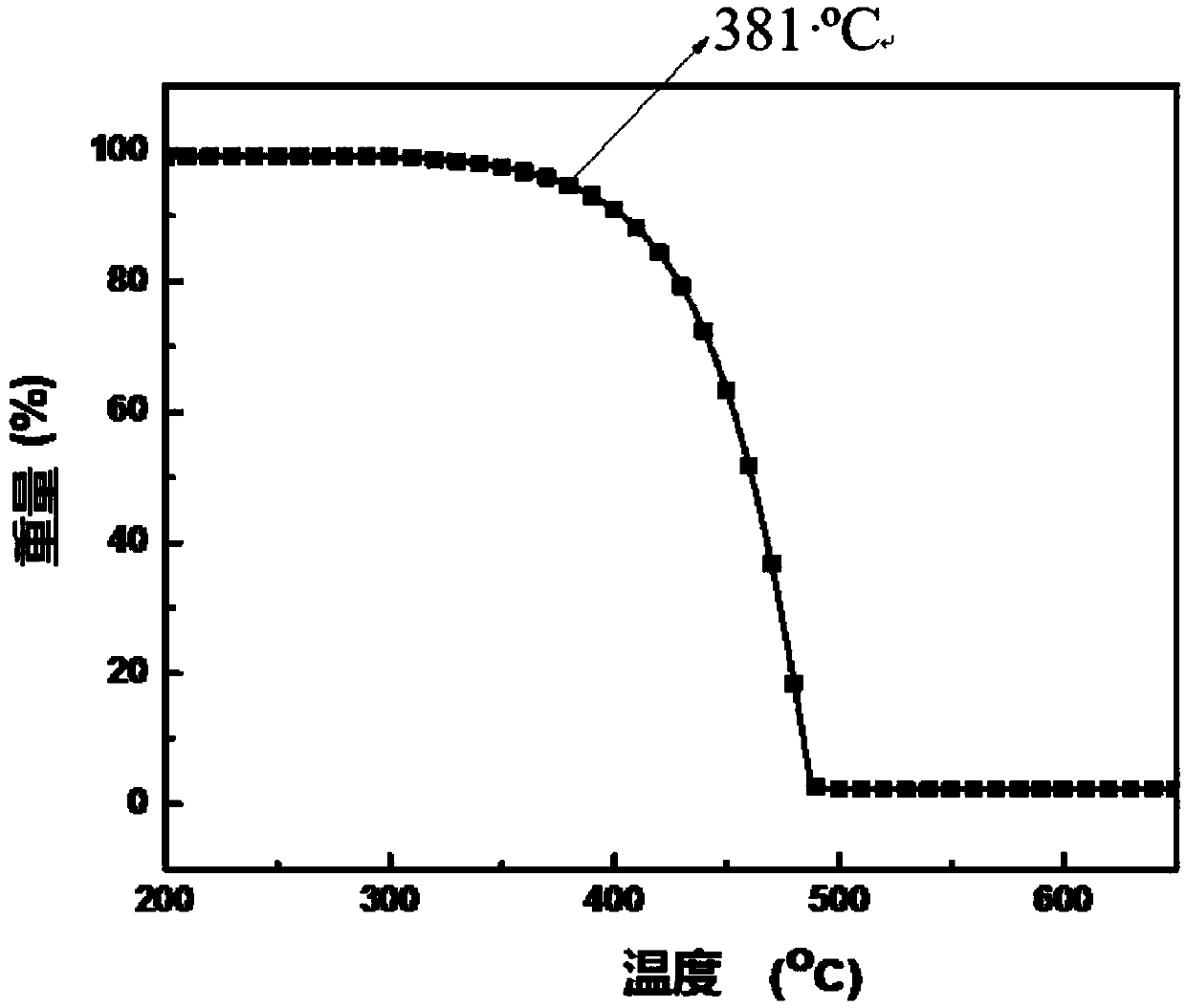
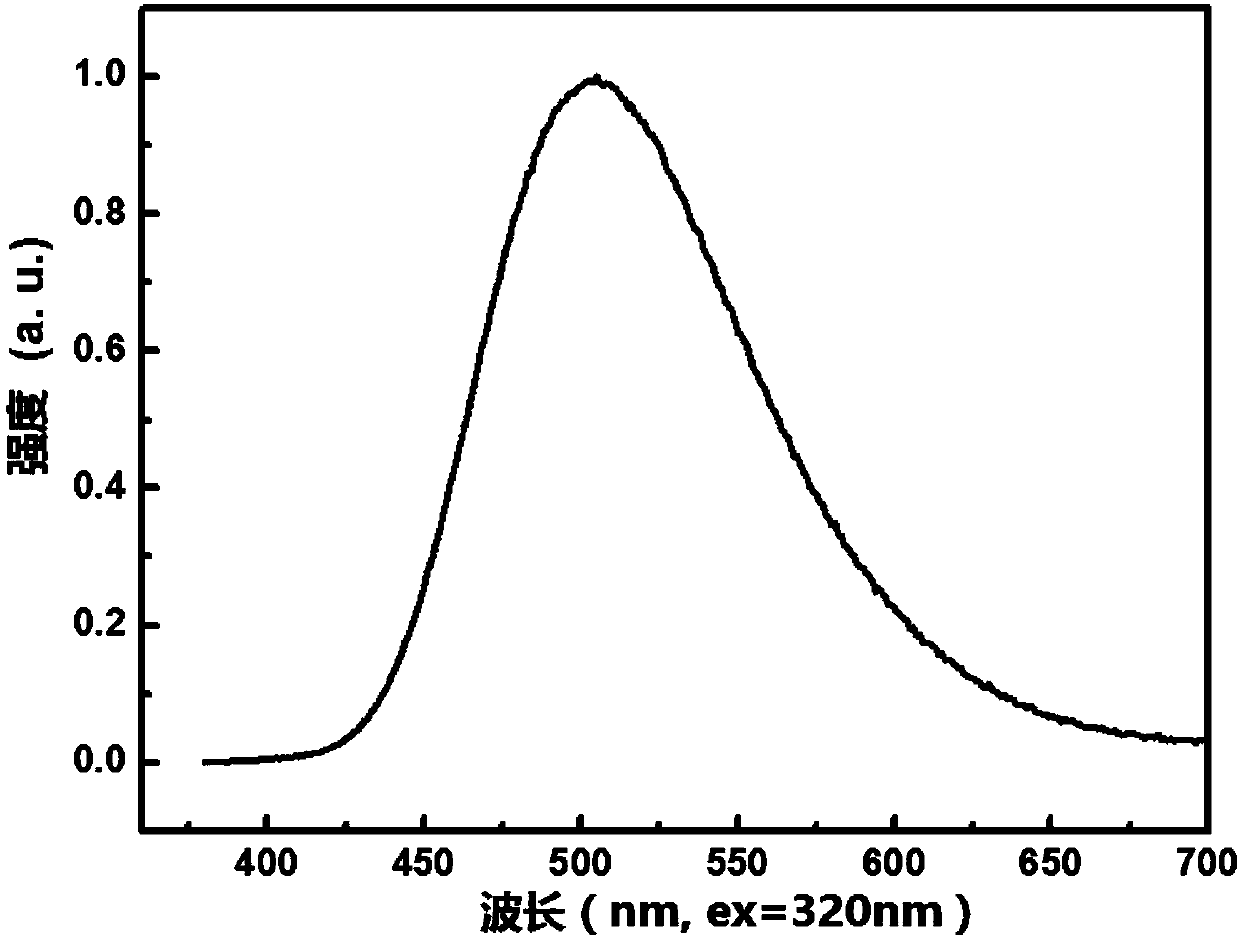
The invention provides a blue fluorescent material and a preparation method thereof. The blue fluorescent material is an organic material, 4'-(8-(4-diphenylamino)phenyl)naphthalene-1-yl)-[1,1'-biphenyl]-4-formonitrile, and has a molecular formula of C43H28N2. The preparation method comprises the following steps: with 4-cyanobiphenyl as an electron acceptor and 4-triphenylamine acid as an electrondonor, carrying out a chemical reaction under catalysis of tetrakis(triphenylphosphine)palladium, thereby obtaining the blue fluorescent material. According to the blue fluorescent material disclosedby the invention, a certain dihedral angle is formed between 4-cyanobiphenyl and 4-triphenylamine acid, so that HOMO and LUMO can realize cross conjugation. Under optical excitation of 320nm, the maximum emission peak is 500nm, and the material emits blue-green light. Meanwhile, the fluorescent material disclosed by the invention has excellent heat stability, and the decomposition temperature is 381 DEG C. The blue fluorescent material disclosed by the invention has excellent heat stability and blue fluorescence emission property and has potential application prospects while serving as a fluorescent material.
Owner:王歧燕
Purple fluorescent material containing tertiary butyl fluorene
ActiveCN103952141AIncreased electron cloud densityHigh densityHydrocarbonsLuminescent compositionsTetrakis(triphenylphosphine)palladium(0)Energy transfer
The invention discloses a purple fluorescent material containing tertiary butyl fluorene and a preparation method thereof. The fluorescent material is characterized in that it is a fluorene organic compound with a molecular formula C37H42; the fluorescent material is synthesized by mixing and reacting 2,7-dibromo-9,9-diethyl fluorene, 4-tert butyl benzoboric acid, tetrakis(triphenylphosphine)palladium and potassium carbonate according to a certain proportion; and the synthesis has simple steps and reaches yield of 60%-92%. Tert butyl benzoboric acid and fluorene are subjected to a cross-coupling reaction to prepare the organic compound with fluorescence properties; and the conjugated big pi bond rich in electrons is conducive to electron transition and energy transfer, so that the compound has good photoelectric activity namely high luminous efficiency, and has potential application prospect.
Owner:湖州优研知识产权服务有限公司
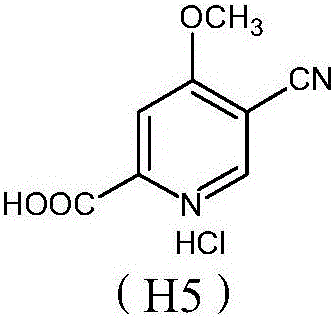
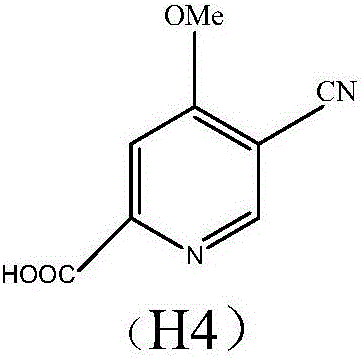
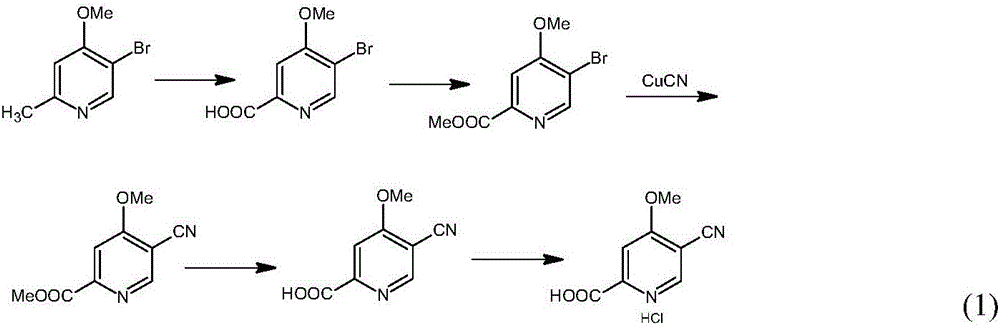
Preparation and application of hydrochlorides of 5-cyano-4-methoxy-2-picolinic acid
ActiveCN106432071AMild reaction conditionsReduce riskOrganic active ingredientsOrganic chemistryTetrakis(triphenylphosphine)palladium(0)Hydrolysis
The invention provides a preparation method of hydrochlorides of 5-cyano-4-methoxyl-2-picolinic acid. According to the preparation method, 5-bromo-4-methoxy-2-methylpyridin is used as raw materials to form 5-bromo-4-methoxyl-2-methyl picolinate sequentially through oxidation and esterification; under the catalysis effect of tetrakis(triphenylphosphine)palladium, the reaction is taken with nucleophilic reagents to obtain the 5-cyano-4-methoxyl-2-methyl picolinate; hydrolysis is performed to obtain 5-cyano-4-methoxy-2-picolinic acid; obtained 5-cyano-4-methoxy-2-picolinic acid reacts with acid to form salts. A novel synthesis path is provided by the invention; a prepared intermediate product of 5-cyano-4-methoxy-2-picolinic acid (H4) can take a reaction with hydrochloric acid under a conventional condition to obtain the hydrochlorides of the 5-cyano-4-methoxy-2-picolinic acid. The intermediate product of active compounds for preparing uretica, natruresis promoting medicine and valuable medicine for treating cardiovascular diseases and diseases due to retention of excessive salts and hydropexis.
Owner:河北正朗制药有限公司
Fluorenyl aromatic phosphine oxide photoelectric material and preparation method thereof
InactiveCN102924516ABlocking interactionBalance injectionGroup 5/15 element organic compoundsSolid-state devicesFluorescenceDiphenylphosphine oxide
The invention relates to a photoelectric material and a preparation method thereof, particularly to a fluorenyl aromatic phosphine oxide photoelectric material and a preparation method thereof, and aims at solving the problem that carrier injection transmission is unbalanced caused by the fact that aromatic hydrocarbon or arylamine functional groups with large conjugated structures are introduced into a fluorine system. The method includes preparing 2-bromine-(9,9-bis-(diphenylphosphine oxide) ) fluorine; and mixing the 2-bromine-(9,9-bis-(diphenylphosphine oxide) ) fluorine, tetrakis (triphenylphosphine) palladium, tetrabutylammonium bromide and borate and dissolving in tetrahydrofuran, adding in NaOH solution, and obtaining the photoelectric material after reaction, extraction, drying and column chromatography; or mixing the 2-bromine-(9,9-bis-(diphenylphosphine oxide) ) fluorine, carbazole, potassium carbonate, copper iodide and 18-crown-6-ether, adding in a high-temperature solvent, and obtaining the photoelectric material after reaction, extraction, drying and column chromatography. The power efficiency of the photoelectric material prepared through the method can reach 2.19lm / W when the material is used for preparing blue-ray fluorescent devices.
Owner:HEILONGJIANG UNIV
Preparation method and application of conjugated molecule based on fluoro-benzo-thiadiazole
InactiveCN108264516ALarge conjugated structureIncrease transfer rateOrganic chemistrySolid-state devicesElectronegativitySolar cell
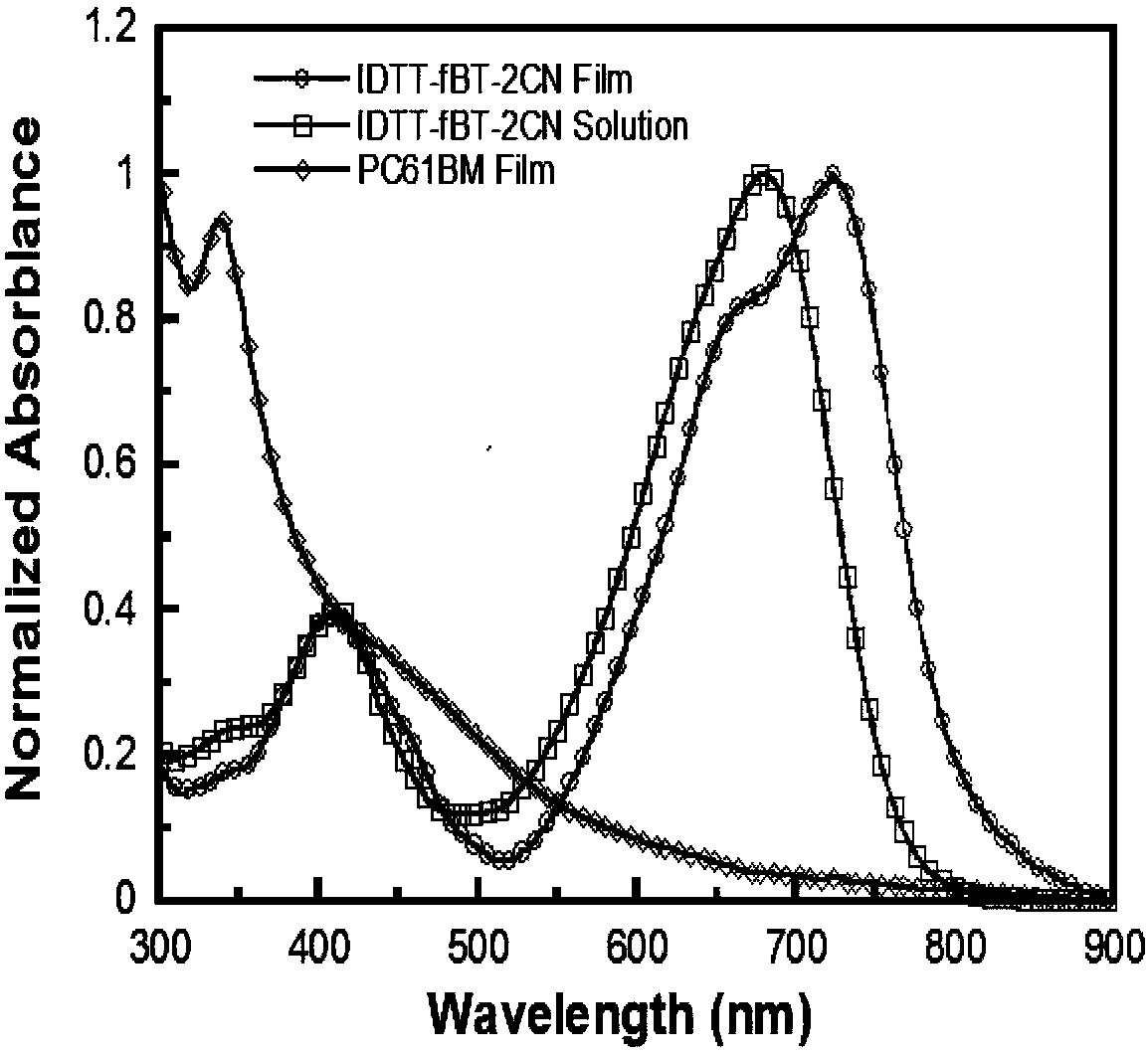
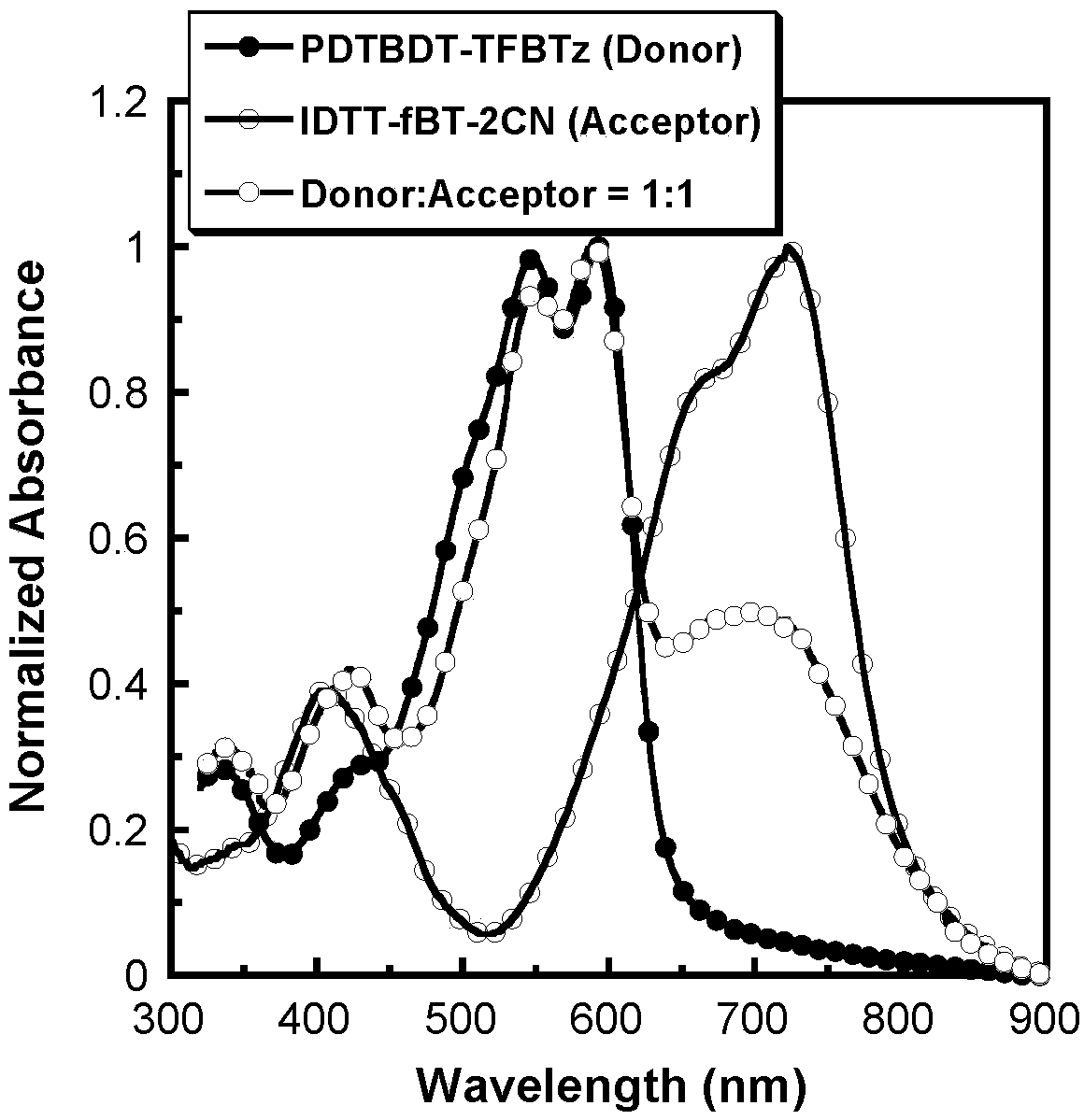
The invention provides a preparation method and an application of a conjugated molecule based on fluoro-benzo-thiadiazole. The preparation method includes the steps: taking methylbenzene as a solvent;taking tetra (triphenylphosphine) palladium as a catalyst; reacting IDTT-fBT (thiophene-fluorobenzothiadiazole) and 2-((6-fluoro-7-benzo[c] [1, 2 and 5] thiadiazole-4-radical) methylene) propane dinitrile at the temperature of 90-110 DEG C to the conjugated molecule based on the fluoro-benzo-thiadiazole. Compared with the prior art, the benzo-thiadiazole has suitable LUMO (lowest unoccupied molecular orbital) and HOMO (highest occupied molecular orbital) energy levels and excellent planarity, so that a conjugated structure of a molecule can be effectively expanded, a fluorine atom is introduced onto a benzene ring, the charge transfer rate in the molecule can be increased as the fluorine atom has high electronegativity, spectrums are absorbed by the fluorine atom, the conjugated moleculecan serve as a receptor material to prepare solar cell devices, and high performance is acquired.
Owner:SHENZHEN SENIOR TECH MATERIAL
A kind of preparation method of phosphine benzene compound
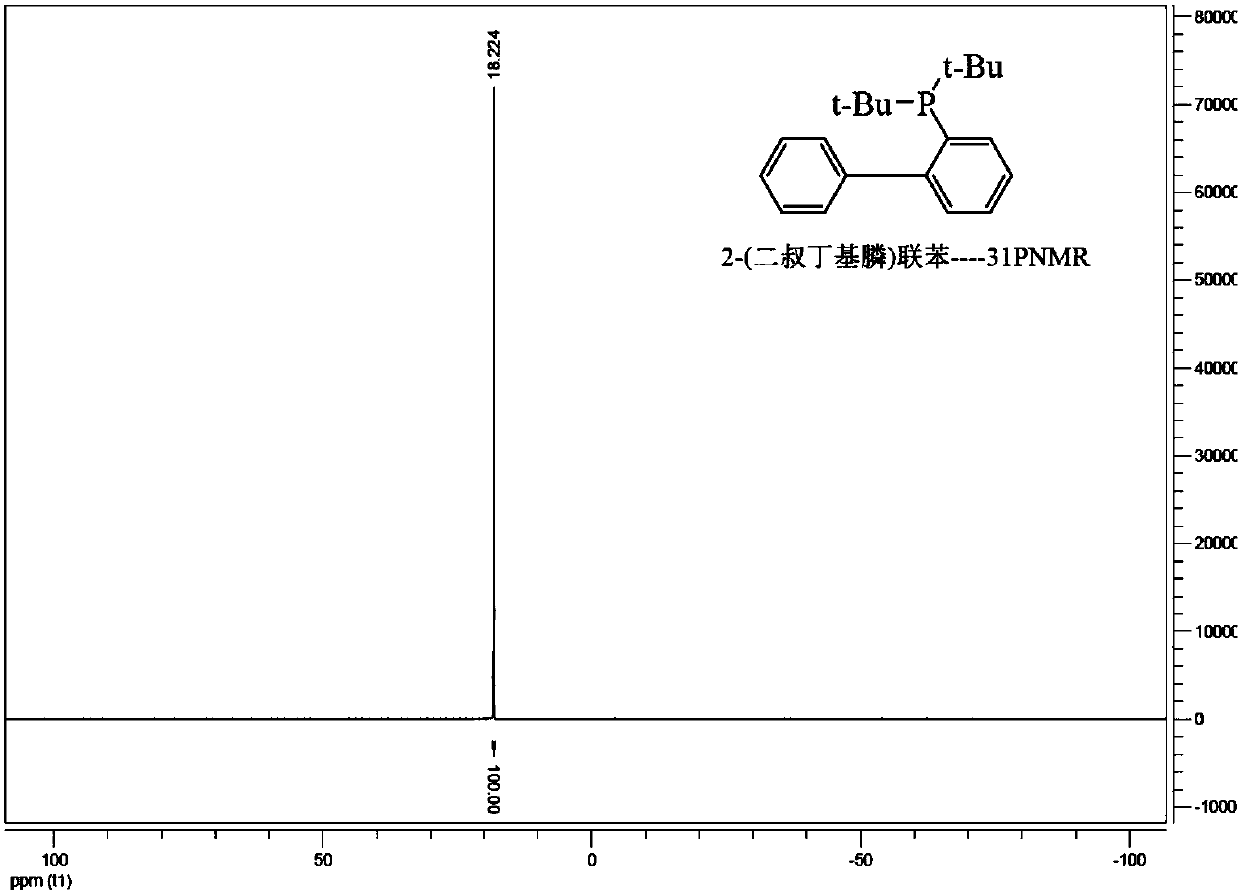
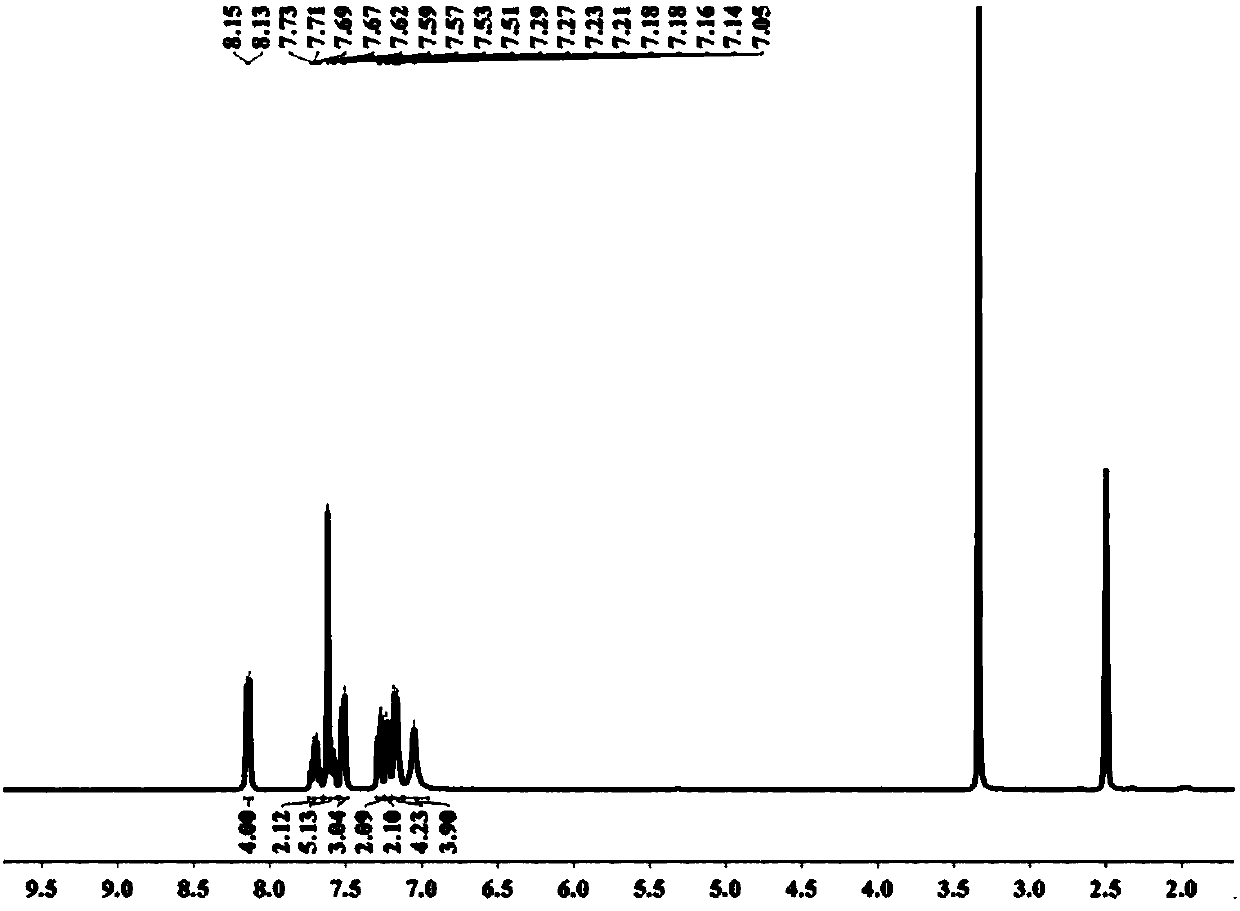
ActiveCN105859774BHigh yieldHigh purityGroup 5/15 element organic compoundsIce waterGrignard reagent
Owner:BEIJING GREENCHEM TECH
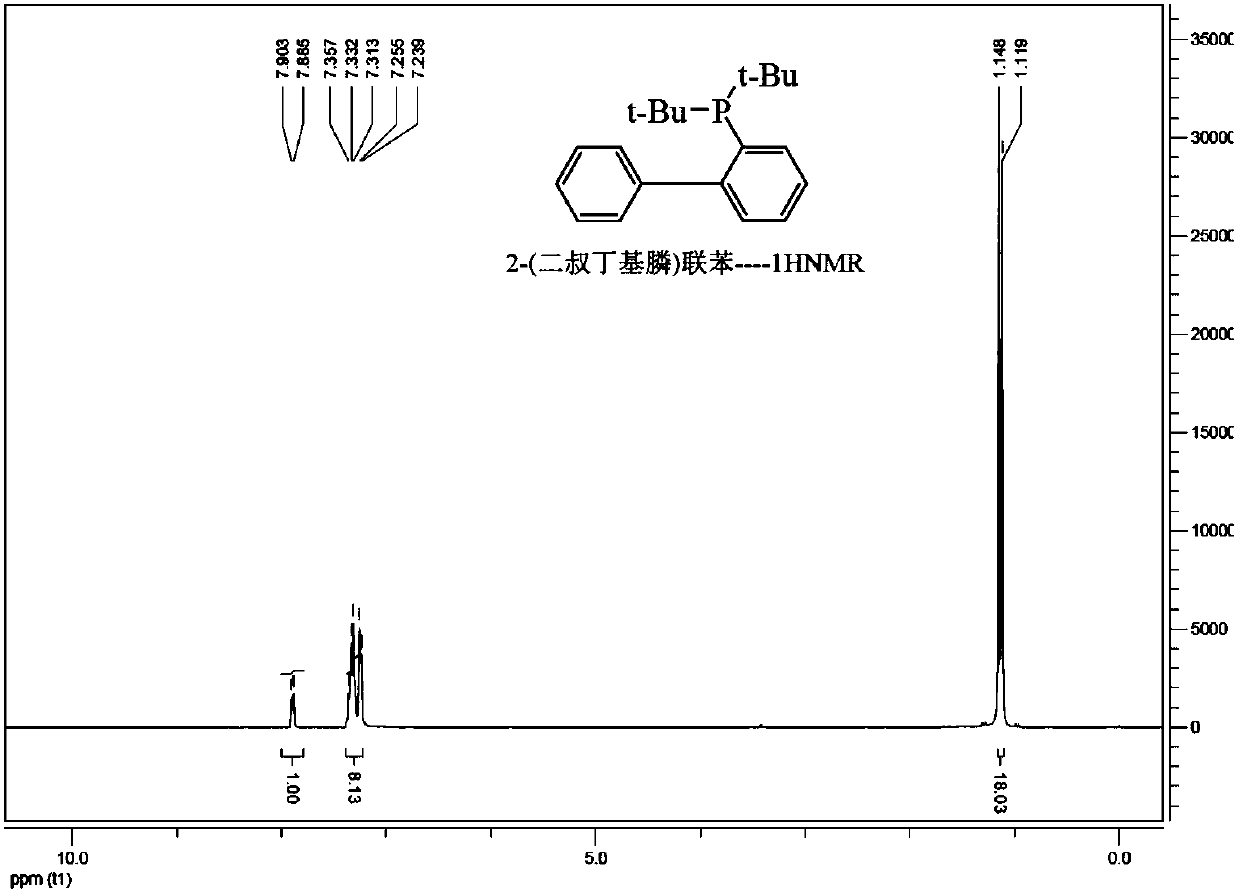
Synthesis process of bis (tri-tert-butylphosphine) palladium (0)
ActiveCN101693725AUniversalSimple processGroup 8/9/10/18 element organic compoundsTetrakis(triphenylphosphine)palladium(0)Reaction temperature
The invention relates to a synthesis process of bis (tri-tert-butylphosphine) palladium (0), which comprises the following steps: reacting in an N,N-dimethyl formamide solvent by taking a palladium substrate and tri-tert-butylphosphine in the mol ratio of 1:2-4 as the main raw materials to generate the bis (tri-tert-butylphosphine) palladium (0) at 20-40 DEG C for 2-4 hours, wherein the reaction formula is as follows: Pd2(dba)3.CHCl3+4P(t-Bu)3, 2Pd[(t-Bu)3P]2+3dba+CHCl3Pd(PPh3)4+2P(t-Bu)3 and Pd[(t-Bu)3P]2+4PPh3. Because tris (dibenzylideneacetone) dipalladium (0) chloroform adducts and tetrakis (triphenylphosphine) palladium (0) are relatively easily obtained raw materials, the process has the characteristics of higher operability, easier commercial production, simple preparation technology, wide raw material sources, low cost, high yield and the like.
Owner:浙江微通催化新材料有限公司
High-temperature-resistant blue fluorescent material and preparation method thereof
InactiveCN107739608AAchieving conjugate blockingImprove thermal stabilityOrganic chemistryLuminescent compositionsChemical reactionElectron donor
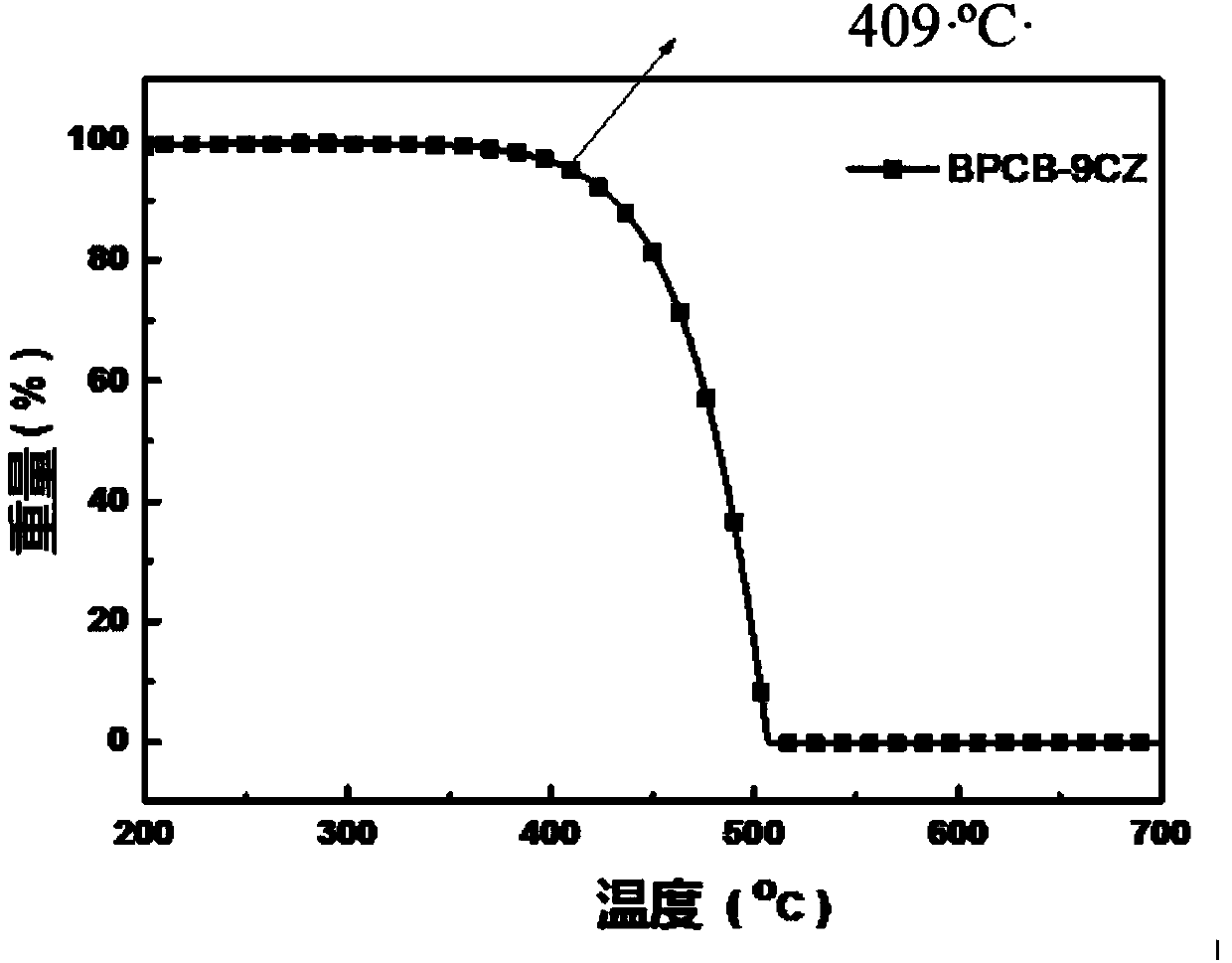
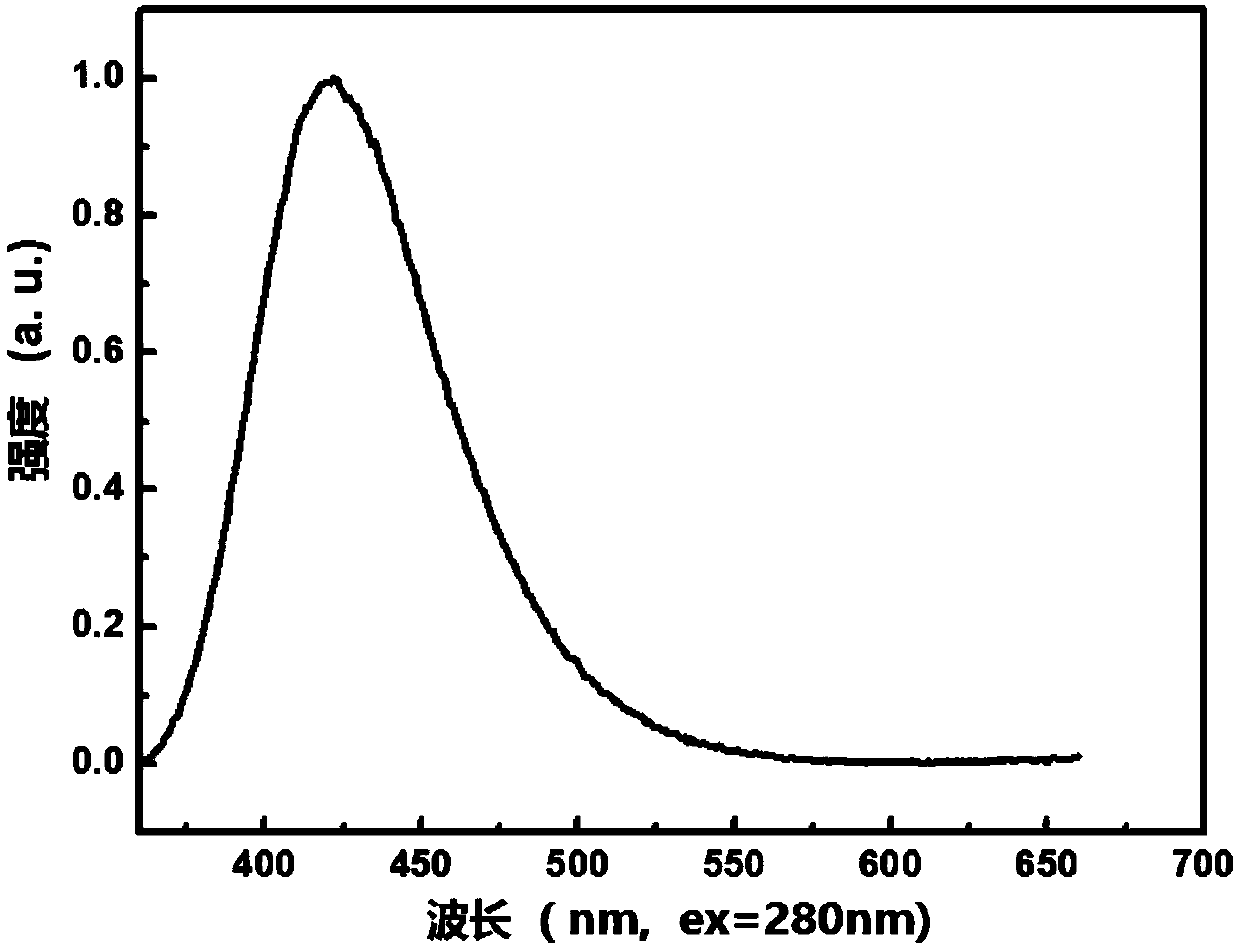
The invention relates to the technical field of organic light emitting materials, in particular to a high-temperature-resistant blue fluorescent material and a preparation method thereof. The high-temperature-resistant blue fluorescent material is an organic material, namely 4'-(8-(4-(9H-carbazole-9-yl) phenyl) naphthalene-1-yl)-[1,1'-biphenyl]-4-formonitrile, the molecular formula of the materialis C41H21N2, 4-cyanobiphenyl is adopted as an electron acceptor, 4-(9H-carbazole-9-yl) phenylboronic acid is adopted as an electron donor, and the material is prepared through chemical reactions under catalysis of tetra(triphenylphosphine) palladium. A certain dihedral angle is formed between 4-cyanobiphenyl and N-phenylcarbazole of the high-temperature-resistant blue fluorescent material provided by the invention, so that conjugate blocking can be achieved for HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital), the maximum emission peak of the material is 440nm under excitation of light of 280nm, and dark blue light is emitted; meanwhile, the fluorescent material provided by the invention is good in thermal stability, and the decomposition temperature of the material is 409 DEG C. The high-temperature-resistant blue fluorescent material provided by the invention is good in thermal stability and blue fluorescent light emission function and has potential application prospects as a fluorescent material.
Owner:王歧燕
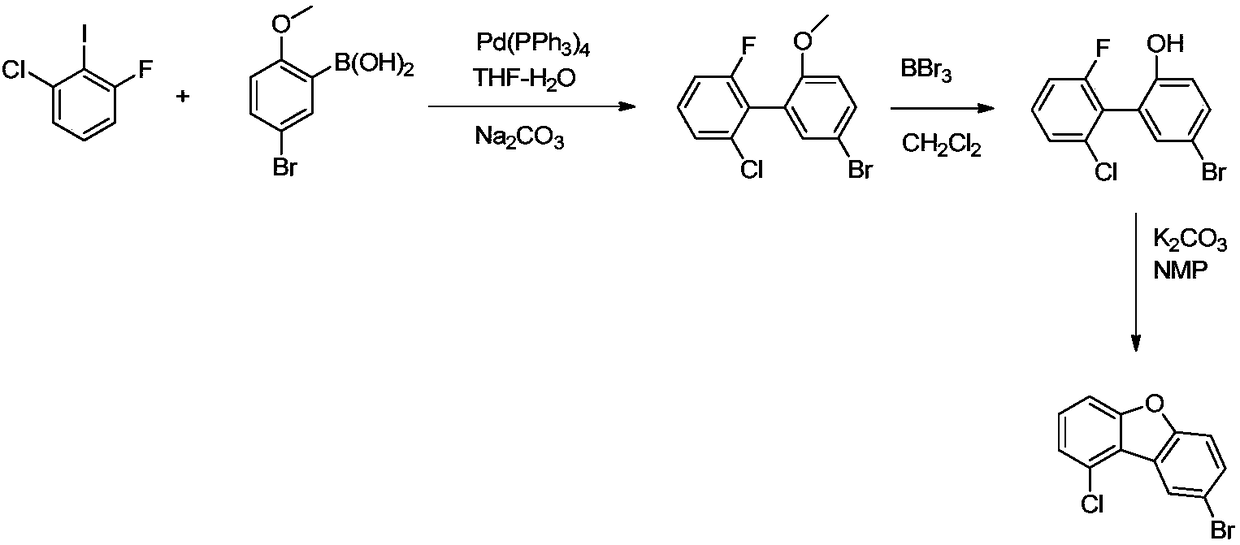
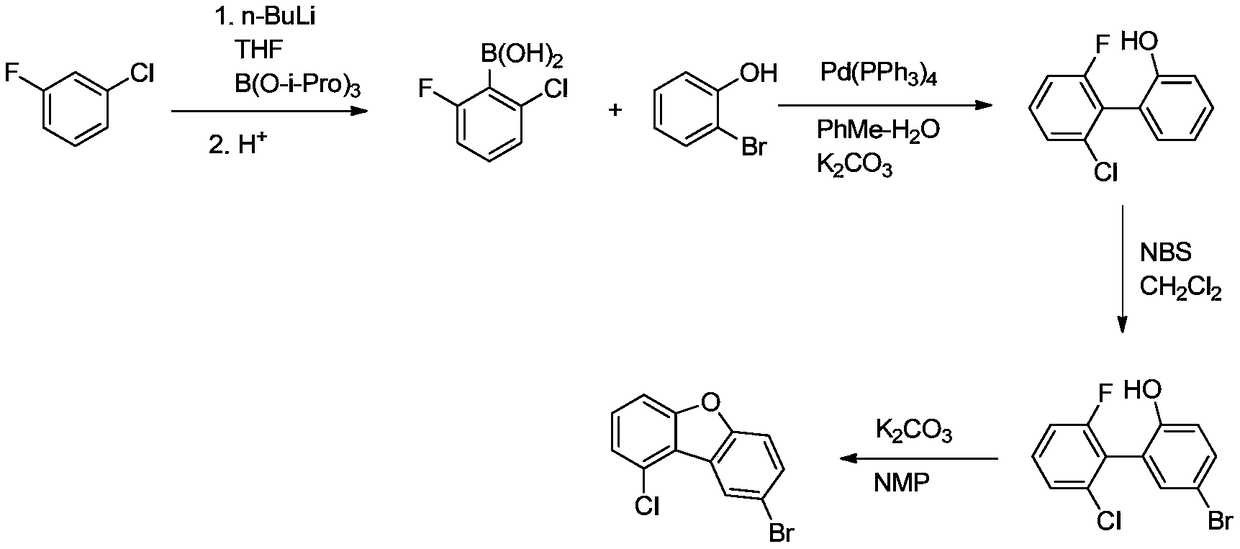
Preparation method of 1'-chloro-8-bromodibenzofuran
InactiveCN109485625AAdvantages of preparation methodAvoid it happening againOrganic chemistryEthyl ChloridePotassium carbonate
The invention discloses an efficient and environment-friendly preparation method of 1'-chloro-8-bromodibenzofuran. The preparation method comprises the following steps: (1) pulling out hydrogen from chloro-3-fluorobenzene first with n-butyllithium, then carrying out a substitution reaction with triisopropyl borate and carrying out acid hydrolysis to obtain 2- fluoro-6-chlorophenylboronic acid; (2)carrying out a reaction between 2- fluoro-6-chlorophenylboronic acid and 2-bromophenol under the action of tetra(triphenylphosphine) palladium and potassium carbonate to obtain 2-fluoro-6-chloro-2'-hydroxy-1,1'-biphenyl; (3) carrying out a reaction between 2-fluoro-6-chloro-2'-hydroxy-1,1'-biphenyl and N-bromosuccinimide to obtain 2-fluoro-6-chloro-2'-hydroxy-5'-bromo-1,1'-biphenyl; and (4) carrying out a ring-closure reaction on 2-fluoro-6-chloro-2'-hydroxy-5'-bromo-1,1'-biphenyl under the action of potassium carbonate to obtain 1'-chloro-8-bromodibenzofuran. Compared with the prior art, thepreparation method can reduce the cost of raw materials and reduce the pollution.
Owner:安徽秀朗新材料科技有限公司
Isoindigo-based narrow band gap donor material and preparation method thereof
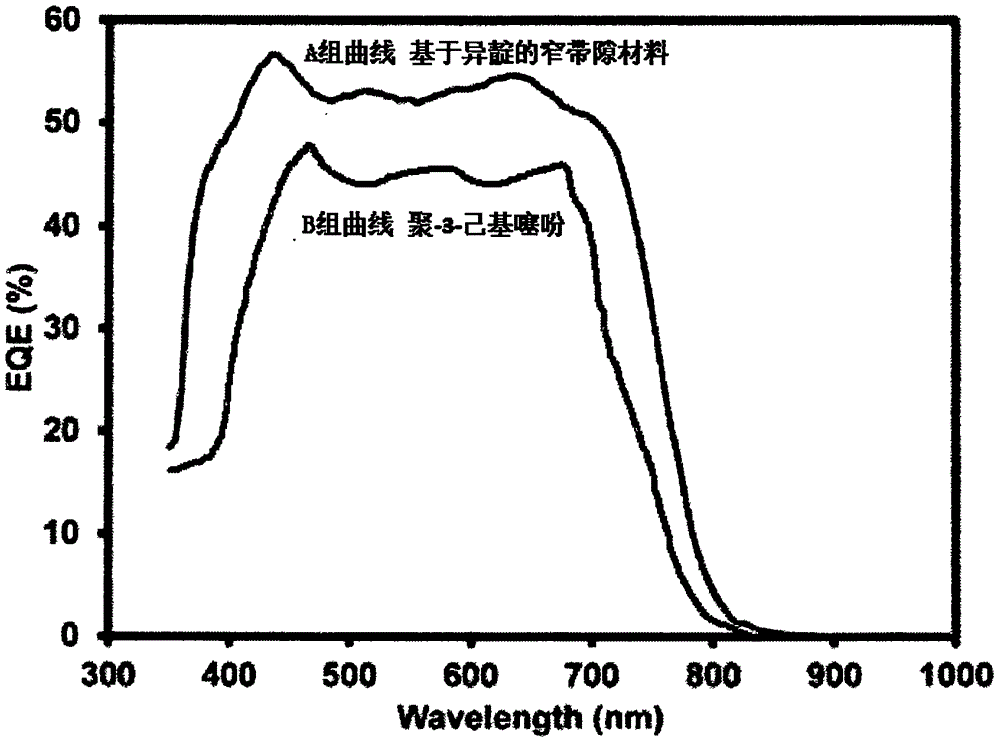
InactiveCN105949231AExcellent short circuit currentImprove photoelectric conversion efficiencyGroup 4/14 element organic compoundsSolid-state devicesOrganic solar cellSolar battery
The invention discloses an isoindigo-based narrow band gap donor material and a preparation method thereof. The method comprises the following steps: 1, mixing a first compound, a second compound, tetrakis(triphenylphosphine)palladium and toluene under the protection of nitrogen, heating the obtained mixture to 80-120DEG C, stirring the heated mixture for 20-30h, and cooling the stirred mixture; and 2, filtering an organic phase obtained in step 1, and drying the filtered organic phase to obtain the narrow band gap donor material. Compared with narrow band gap donor materials in the prior art, the narrow band gap donor material prepared in the invention contains isoindigo and thiophene units, and has excellent short circuit current, photoelectric conversion efficiency and external quantum efficiency when applied to organic solar batteries. The narrow band gap donor material prepared in the invention is a low molecular material, has good dissolvability, can be easily dissolved in organic solvents and coated, and is suitable for processing technologies of the organic solar batteries.
Owner:NINGBO XIAYUAN TECH
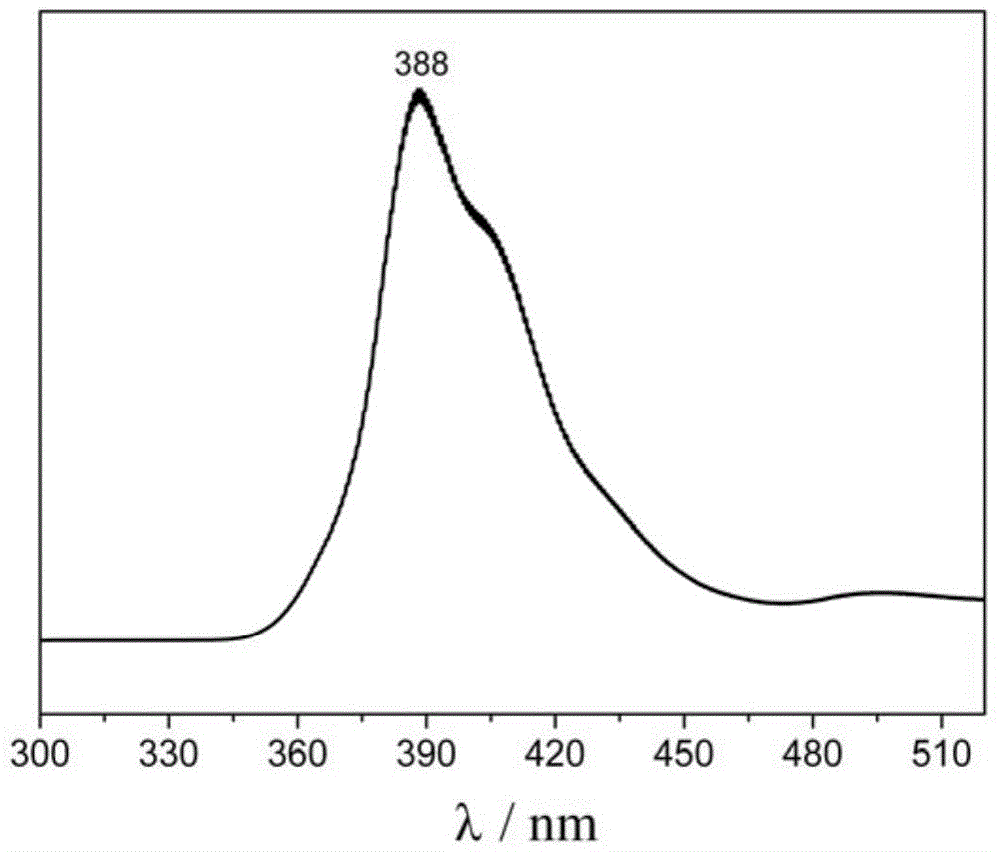
Method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene
The invention discloses a method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene, which comprises the following steps: putting chlorobenzene and butanedioic anhydride into a reaction bulb, sufficiently stirring, adding HB(OH)2, heating to 40-50 DEG C, stirring uniformly, and standing to room temperature; adding 7.26g of 2-bromobenzaldehyde and 45mL of ethanol into the reaction bulb twice, stirring uniformly, adding 86.0g of 20% sodium carbonate water solution, quickly adding tetra(triphenylphosphine) palladium, heating from room temperature to 70-80 DEG C (under reflux), keeping stirring at such temperature to react for 6 hours, sampling, and carrying out HPLC (high performance liquid chromatography) central control; after the 2-bromobenzaldehyde is reacted completely, cooling the reaction solution to 25-30 DEG C, and dropwisely adding methanesulfonic acid at room temperature; and after the reaction finishes, adding potassium carbonate into the reaction bulb, carrying out phase splitting, filtering and precipitating the solid powder, wherein the yield is 60.12%, and the HPLC is 99.12%. The method avoids the problems of complex synthesis, incapability of mass production, environmental pollution, side reactions and the like in the traditional method.
Owner:JINING XINRUIDA INFORMATION TECH CO LTD
A purple fluorescent material containing tert-butylfluorene
ActiveCN103952141BIncreased electron cloud densityHigh densityHydrocarbonsLuminescent compositionsEnergy transferSimple Organic Compounds
The invention discloses a purple fluorescent material containing tertiary butyl fluorene and a preparation method thereof. The fluorescent material is characterized in that it is a fluorene organic compound with a molecular formula C37H42; the fluorescent material is synthesized by mixing and reacting 2,7-dibromo-9,9-diethyl fluorene, 4-tert butyl benzoboric acid, tetrakis(triphenylphosphine)palladium and potassium carbonate according to a certain proportion; and the synthesis has simple steps and reaches yield of 60%-92%. Tert butyl benzoboric acid and fluorene are subjected to a cross-coupling reaction to prepare the organic compound with fluorescence properties; and the conjugated big pi bond rich in electrons is conducive to electron transition and energy transfer, so that the compound has good photoelectric activity namely high luminous efficiency, and has potential application prospect.
Owner:湖州优研知识产权服务有限公司
Method for recycling palladium from tetra(triphenylphosphine) palladium waste liquid
ActiveCN113151693AWon't happenCreate pollutionTetrakis(triphenylphosphine)palladium(0)Oxidizing agent
The invention discloses a method for recycling palladium from tetra(triphenylphosphine) palladium waste liquid. The method comprises the following steps: (1), adding 10% by volume of ethanol into a certain amount of tetra(triphenylphosphine) palladium waste liquid, heating to 120 DEG C, concentrating for one hour, cooling to room temperature to generate a small amount of floccule precipitate, then filtering, collecting solids and retaining filtrate; (2), adding a certain amount of oxidizing agent into the filtrate, then adding a complexing reagent to generate a small amount of floccules, filtering, and discharging the filtrate; and (3) combining the previously collected solids, and then roasting, reducing and refining to obtain high-purity palladium powder. The method is simple in operation condition, low in equipment requirement, mild in reaction condition, excellent in safety and high in recovery rate; the palladium content in the treated waste liquid is less than 5ppm; and the waste liquid can be discharged. According to the method for recycling palladium from tetra(triphenylphosphine) palladium waste liquid, the recovery rate of the palladium powder is greater than 99.5%, and the purity of the palladium powder is higher than 99.95%; and the method is obvious in economic and environmental protection benefits.
Owner:SINO PLATINUM METALS CO LTD
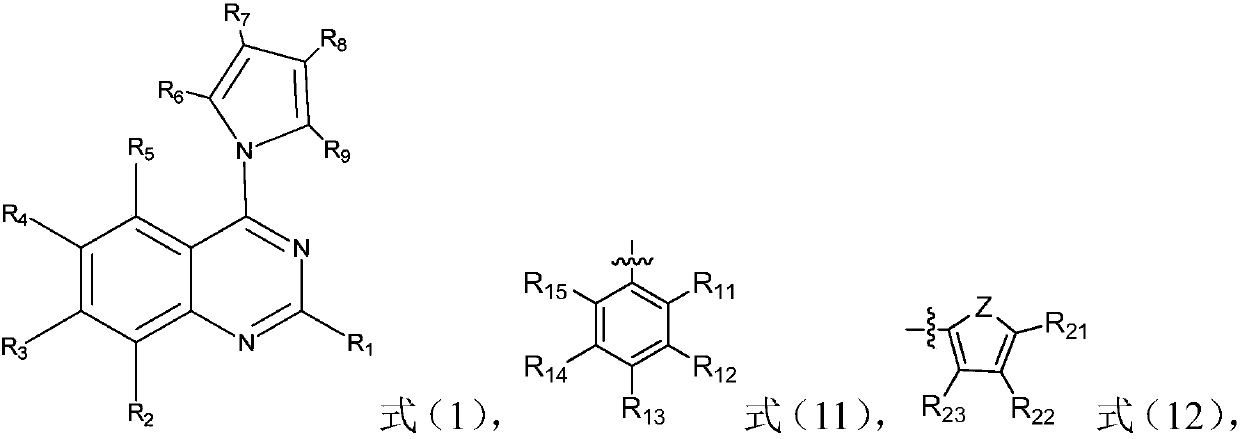
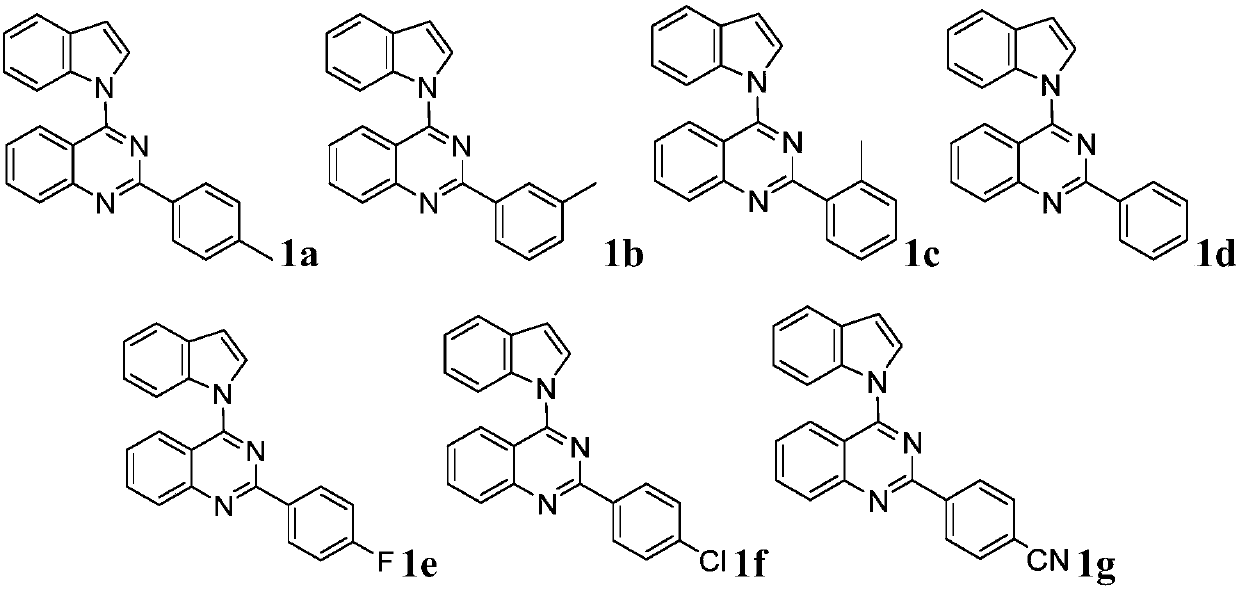
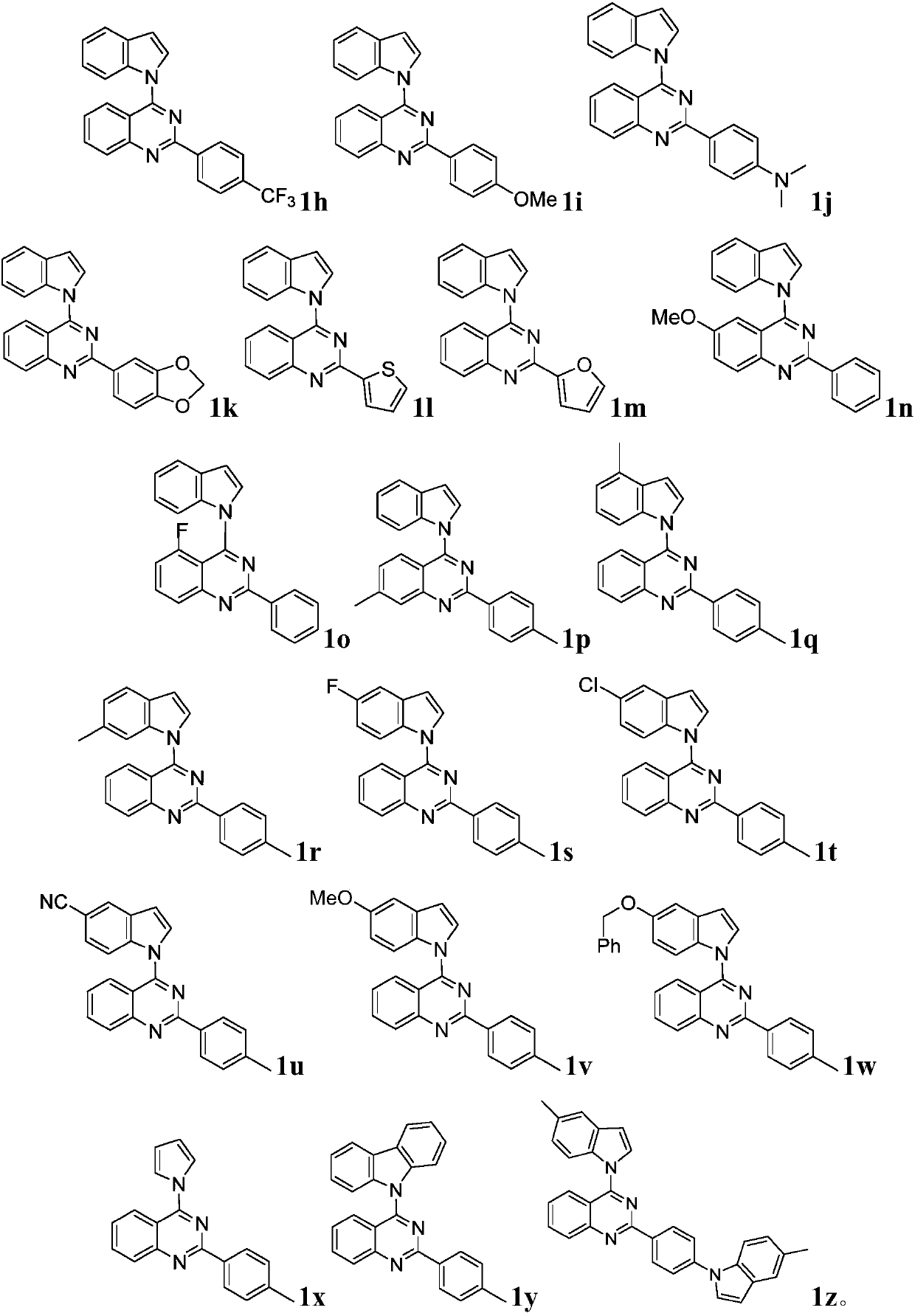
Quinazoline derivative and preparation method and application thereof
ActiveCN109096254AHigh antibacterial activityGood antitumor activityOrganic chemistryAntiinfectivesTetrakis(triphenylphosphine)palladium(0)Trifluoroacetic acid
Owner:JIANGXI NORMAL UNIV
A method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene
The invention discloses a method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene, which comprises the following steps: putting chlorobenzene and butanedioic anhydride into a reaction bulb, sufficiently stirring, adding HB(OH)2, heating to 40-50 DEG C, stirring uniformly, and standing to room temperature; adding 7.26g of 2-bromobenzaldehyde and 45mL of ethanol into the reaction bulb twice, stirring uniformly, adding 86.0g of 20% sodium carbonate water solution, quickly adding tetra(triphenylphosphine) palladium, heating from room temperature to 70-80 DEG C (under reflux), keeping stirring at such temperature to react for 6 hours, sampling, and carrying out HPLC (high performance liquid chromatography) central control; after the 2-bromobenzaldehyde is reacted completely, cooling the reaction solution to 25-30 DEG C, and dropwisely adding methanesulfonic acid at room temperature; and after the reaction finishes, adding potassium carbonate into the reaction bulb, carrying out phase splitting, filtering and precipitating the solid powder, wherein the yield is 60.12%, and the HPLC is 99.12%. The method avoids the problems of complex synthesis, incapability of mass production, environmental pollution, side reactions and the like in the traditional method.
Owner:JINING XINRUIDA INFORMATION TECH CO LTD
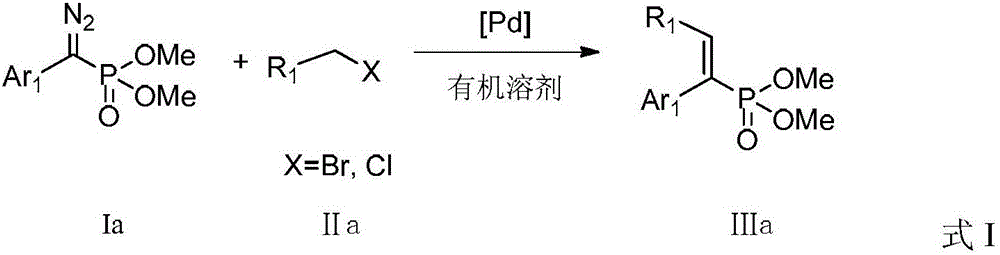
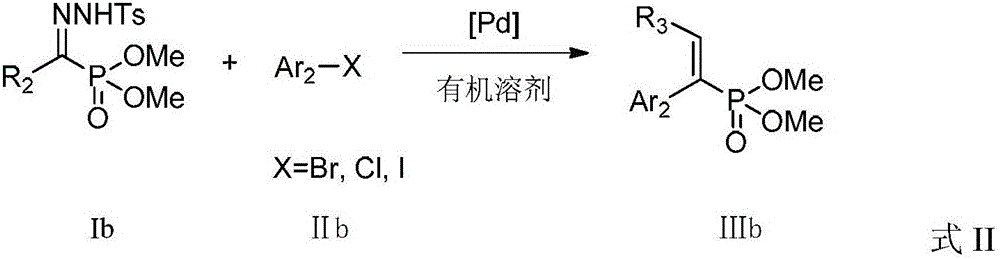
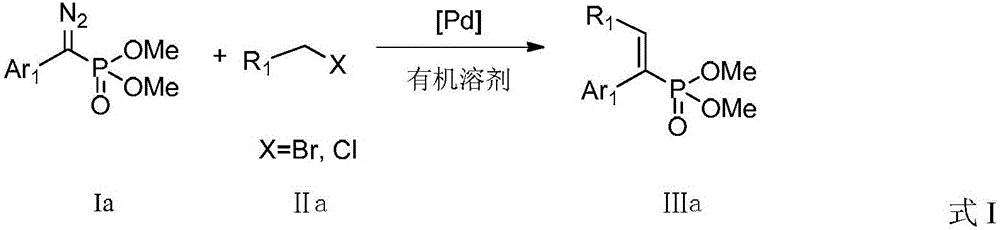
A kind of preparation method of alkenyl phosphonate compound
ActiveCN104926867BLower reaction costImprove reaction efficiencyGroup 5/15 element organic compoundsTetrakis(triphenylphosphine)palladium(0)Aryl
The invention discloses a preparation method for an alkenyl phosphate compound. By taking palladium acetate or tetra(triphenylphosphine) palladium as a catalyst, an alkali, a ligand, a diazo compound and a halide or an alkali, tosylhydrazone and a halide react under protection of an N2 atmosphere in an organic solvent to obtain the alkenyl phosphate compound RR'C=C(Ar)(P(O)(OMe)2), wherein Ar represents substituted or unsubstituted aryl comprising phenyl, naphthyl and heterocyclic rings; R and R' represent substituted or unsubstituted aryl, alkyl, alkenyl or hydrogen. According to the reaction referred to the invention, an E-configuration alkenyl phosphate compound can be smoothly obtained. The preparation method is convenient and simple to operate and has good tolerance and universality on functional groups. Moreover, the use level of the catalyst is relatively small, neither the reagent nor the solvent is specially treated, and the reaction cost is relatively low, so that the preparation method can be widely used for preparing the alkenyl phosphate compound.
Owner:PEKING UNIV
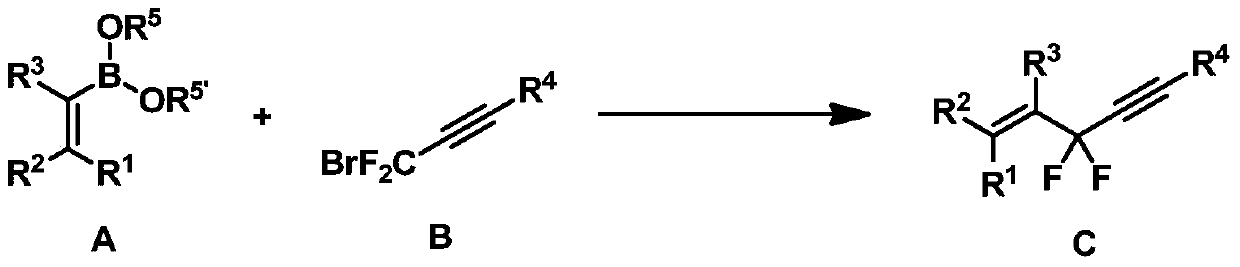
Difluoropropargyl-containing compound, preparation method and application
ActiveCN105272997BReduce dosageSimple and fast operationSilicon organic compoundsCarboxylic acid nitrile preparationCompound cAdduct
The present invention discloses a difluoropropargyl-containing compound, a preparation method and applications. The preparation method comprises that in the presence of an organic solvent, a catalyst, a ligand and an alkali, a compound A and a compound B are subjected to a coupling reaction to obtain a compound C, wherein the catalyst is one or a plurality of materials selected from palladium acetate, palladium trifluoroacetate, tetrakis(triphenylphosphine)palladium, tris(dibenzylideneacetone)dipalladium chloroform adduct, and tris(dibenzylideneacetone)dipalladium, and the ligand is one or a plurality of materials selected from triphenylphosphine, tris(o-methoxyphenyl)phosphine, tris(o-methylphenyl)phosphine, tri-tert-butylphosphine fluoroborate, 2-(dicyclohexylphosphino)-2'-methylbiphenyl, 2-(di-tert-butylphosphino)biphenyl, and 4,5-bisdiphenylphosphino-9,9-dimethyl xanthene. According to the present invention, the method has characteristics of low catalyst consumption, mild reaction condition, high region selectivity, wide application range, and high reaction yield, and is suitable for industrial production. The compounds A, B and C are defined in the specification.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Selenophene vinyl compounds and organic conjugated material thereof, and preparation method of organic conjugated material
InactiveCN105859681AThe synthetic route is simpleFew synthetic stepsOrganic chemistryTetrakis(triphenylphosphine)palladium(0)Organic solvent
The embodiment of the invention relates to selenophene vinyl compounds and an organic conjugated material thereof, and a preparation method of the organic conjugated material. The structural general formula of the selenophene vinyl compounds is disclosed in the specification, wherein Et is ethyl. The structural general formula of the organic conjugated material of the selenophene vinyl compounds is disclosed in the specification, wherein A is an aromatic conjugated unit with different electron donating and receiving capacities, n is polymerization degree and is any whole number ranging from 2 to 100. The selenophene vinyl compounds are prepared from selenophene, n-butyllithium, hexacarbonyl chromium, triethoxytetrafluoro borate and tetra(triphenylphosphine) palladium by olefin double-decomposition reaction in an organic solvent. The corresponding organic conjugated material is prepared by still coupled reaction. The preparation method provided by the invention has the advantages of simple route, fewer steps and low production cost.
Owner:UNIVERSITY OF CHINESE ACADEMY OF SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
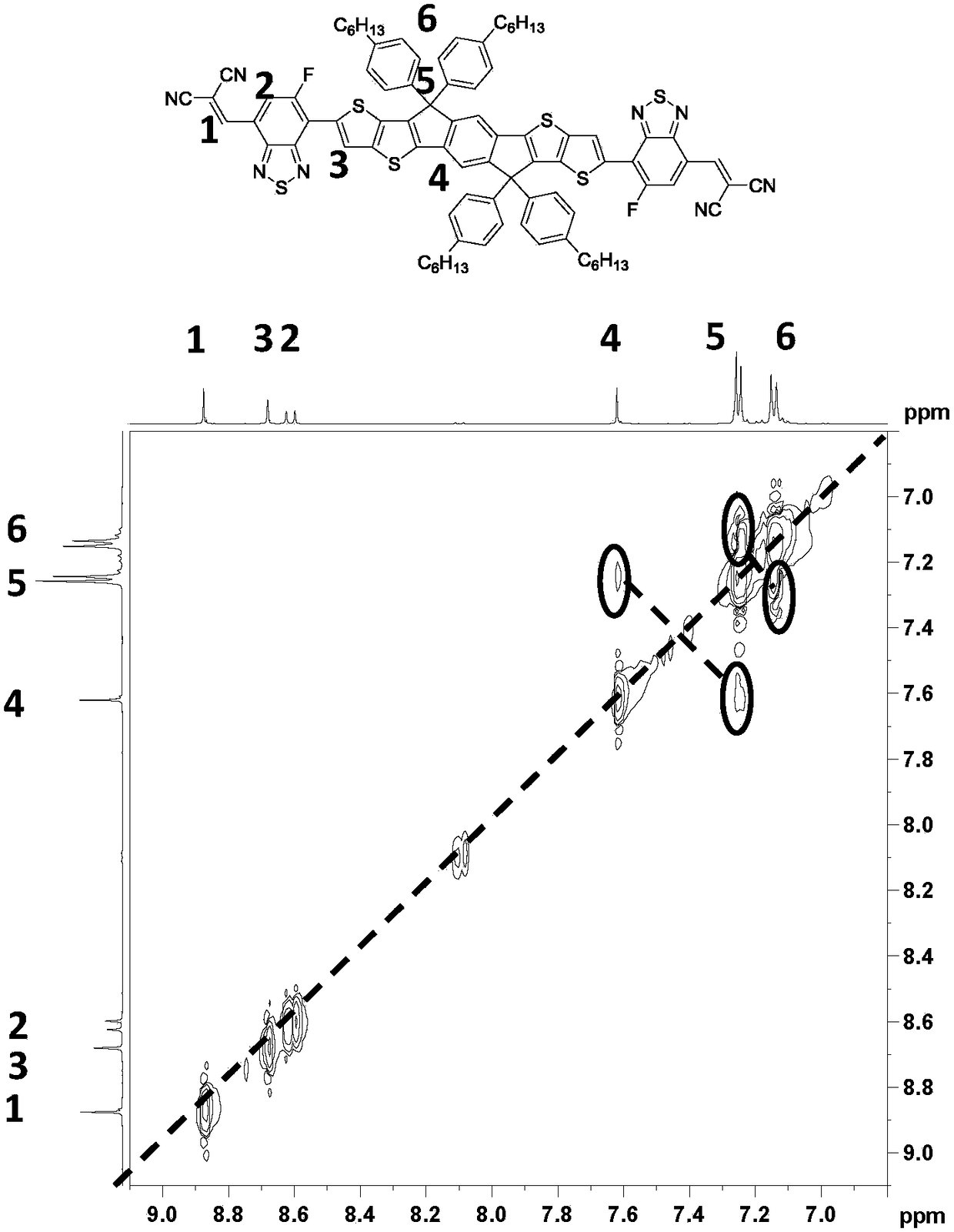
![Method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene Method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene](https://images-eureka.patsnap.com/patent_img/f4fc7d5f-7893-4a9f-926e-8a805d297d20/BDA0000595989170000021.PNG)
![A method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene A method for synthesizing 1-chloro-2-bromobenzo[9,10]phenanthrene](https://images-eureka.patsnap.com/patent_img/d2c66c83-f315-4117-ad6e-14da904732ab/GDA0000807204590000021.PNG)