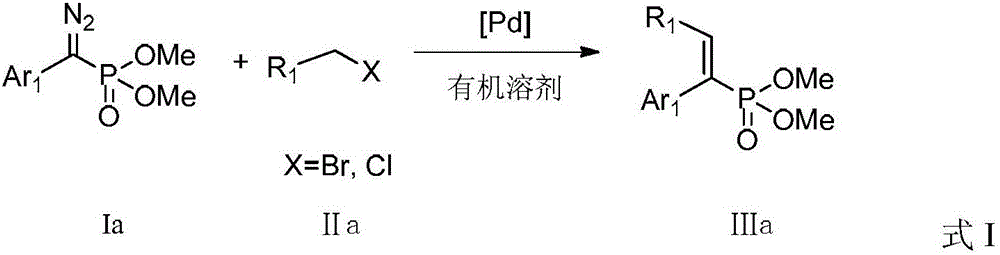
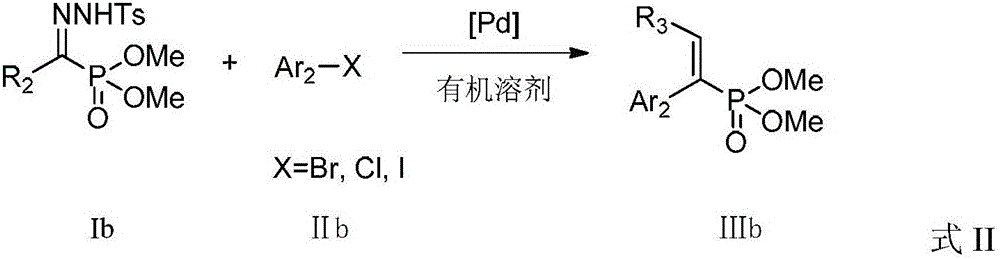
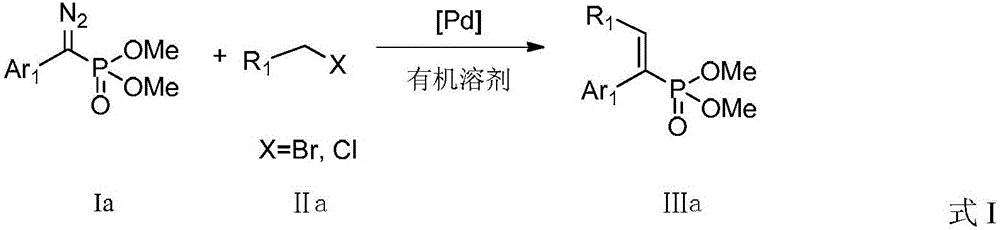
A kind of preparation method of alkenyl phosphonate compound
A technology of alkenyl phosphonate and compounds, which is applied in the field of preparation of alkenyl phosphonate compounds, can solve problems such as poor regioselectivity, harsh reaction conditions, and limited substrate range, and achieve good tolerance, low reaction cost, The effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of (E)-1,2-Diphenylvinyl Phosphate Dimethyl Ester
[0031] Add 45.2 mg (i.e. 0.20 mmol) dimethyl diazo (phenylmethyl) phosphate, 9.2 mg tris (2-furyl) phosphine (i.e. diazo (phenyl methyl) phosphate di 20mol% of methyl ester), then weighed 2.2mg (5mol% of diazo (phenylmethyl) dimethyl phosphate) palladium acetate, replaced the system with nitrogen environment after the reaction tube was sealed, and added 2mL of toluene , and then added 40.4 mg of diisopropylamine (ie 0.40 mmol), and finally added 42.8 mg (ie 0.25 mmol) of benzyl bromide, and reacted at 80° C. for 5 hours. After the reaction, filter, rinse with ethyl acetate, concentrate, and purify by thin-plate chromatography with an eluent with a volume ratio of petroleum ether:ethyl acetate of 1:1 to obtain (E)-1,2-diphenylvinyl phosphoric acid Dimethyl ester, the compound is a colorless liquid with a yield of 88%. When the reaction temperature is 100°C, the yield is 62%; when the reaction temperature is ...
Embodiment 2
[0036] Synthesis of (E)-((1-phenyl)(2-(4-methylphenyl))vinyl)phosphate dimethyl ester
[0037]Add 45.2 mg (i.e. 0.20 mmol) dimethyl diazo (phenylmethyl) phosphate, 9.3 mg tris (2-furyl) phosphine (i.e. diazo (phenyl methyl) phosphate di 20mol% of methyl ester), then weighed 2.2mg (ie 5mol% of diazo (phenylmethyl) dimethyl phosphate) palladium acetate, replaced the inside of the system with nitrogen atmosphere after the reaction tube was sealed, and added 2mL of toluene , and then added 40.4 mg of diisopropylamine (ie 0.40 mmol), and finally added 46.3 mg (ie 0.25 mmol) of 4-methylbenzyl bromide, and reacted at 80° C. for 5 hours. After the reaction, it was filtered, washed with ethyl acetate and concentrated, and purified by thin-plate chromatography with petroleum ether: ethyl acetate volume ratio of 1:1 eluent to obtain (E)-((1-phenyl)(2- (4-methylphenyl)) vinyl) dimethyl phosphate, its structure is shown in the following formula:
[0038]
[0039] The compound is a col...
Embodiment 3
[0042] Synthesis of (E)-((1-phenyl)(2-(4-phenylphenyl))vinyl)phosphate dimethyl ester
[0043] Add 45.2 mg (i.e. 0.20 mmol) dimethyl diazo (phenylmethyl) phosphate, 9.3 mg tris (2-furyl) phosphine (i.e. diazo (phenyl methyl) phosphate di 20mol% of methyl ester), then weighed 2.2mg (5mol% of diazo (phenylmethyl) dimethyl phosphate) palladium acetate, and finally added 61.8mg (0.25mmol) of 4-phenylbenzyl bromide After sealing the reaction tube, replace the system with a nitrogen atmosphere, add 2 mL of toluene, and then add 40.4 mg of diisopropylamine (0.40 mmol), and react at 80° C. for 5 hours. After the reaction, it was filtered, washed with ethyl acetate and concentrated, and purified by thin-plate chromatography with petroleum ether: ethyl acetate volume ratio of 1:1 eluent to obtain (E)-((1-phenyl)(2- (4-phenylphenyl)) vinyl) dimethyl phosphate, its structure is shown in the following formula:
[0044]
[0045] The compound is a colorless liquid with a yield of 76%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com