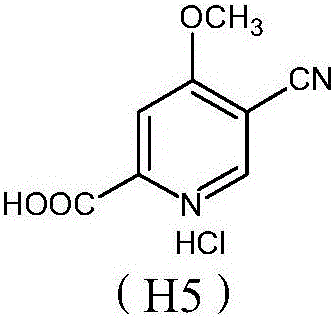
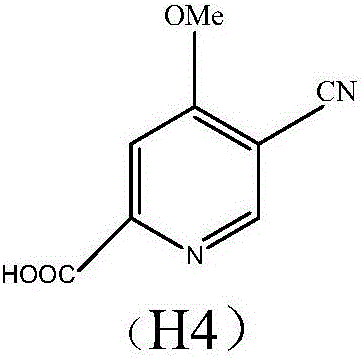
Preparation and application of hydrochlorides of 5-cyano-4-methoxy-2-picolinic acid
A technology for pyridinecarboxylic acid hydrochloride and picolinic acid, which is applied in the field of heterocyclic compound pharmaceutical intermediates and its preparation, can solve the problems of unreported synthesis process routes of compounds, and achieve reduced risks, simple synthesis routes and excellent reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
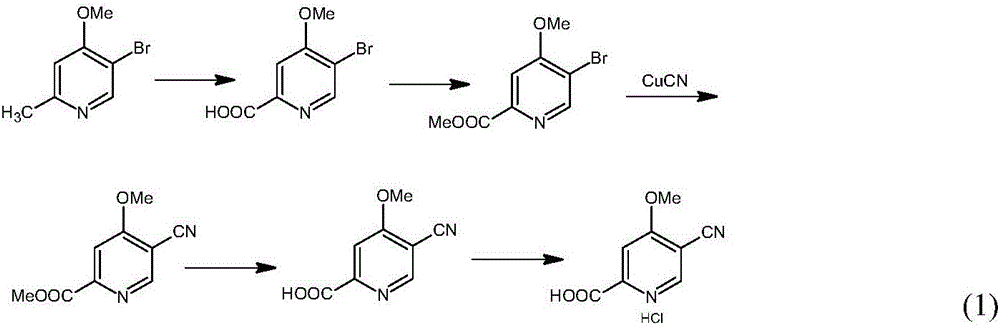
[0036] In the embodiments, unless otherwise specified, the means used are conventional technical means in the art. Example 1. Synthesis of 5-bromo-4-methoxy-2-pyridinecarboxylic acid (H1) by 5-bromo-4-methoxy-2-picoline (H0)
[0037] Its synthesis method is as follows:
[0038]
[0039] In the there-necked flask, install a mechanical stirrer and a thermometer, dissolve 10 grams (0.05mol) of 5-bromo-4-methoxy-2-methylpyridine in 100ml of acetone solution, cool down to 0°C, and slowly add 5% KMnO 4 100ml of aqueous solution, kept at 0°C for 2 hours, continued to react for 16 hours when the temperature was raised to room temperature, and detected the end point of the reaction with TLC until 5-bromo-4-methoxy-2-methylpyridine disappeared, and added 20ml of ethyl alcohol Diol terminated the reaction, continued to stir for 30min, and filtered to remove MnO 2 solid, the reaction solution was concentrated to dryness, and 100ml of 5% NaHCO was added 3 Make it dissolve completely...
Embodiment 2
[0042] Example 2. Synthesis of 5-bromo-4-methoxy-2-pyridinecarboxylic acid methyl ester (H2) from 5-bromo-4-methoxy-2-pyridinecarboxylic acid (H1)
[0043] Its synthesis method is as follows:
[0044]
[0045] In the there-necked flask, install a mechanical stirrer and a thermometer, dissolve 20 grams (0.05mol) of 5-bromo-4-methoxy-2-pyridinecarboxylic acid (H1) in 200mL of methanol, add 10ml of sulfuric acid (mass fraction 98%) Concentrated sulfuric acid), heat up to 60-70°C, keep warm and continue to react for 16 hours, detect with TLC until the raw material disappears, stop the reaction, concentrate to dryness, add 100ml of ethyl acetate, add 50ml of 5% NaHCO 3 Neutralize, wash with water until neutral, dry with anhydrous sodium sulfate, filter, concentrate, and crystallize with a mixture of toluene and ethyl acetate to obtain an off-white solid with a yield of 95%. The structure of the compound is characterized by NMR.
[0046] 1 HNMR (CDCl 3 ): σ9.23ppm (1H), σ8.48p...
Embodiment 3
[0048] Example 3. Synthesis of 5-cyano-4-methoxy-2-pyridinecarboxylic acid methyl ester (H3) from 5-bromo-4-methoxyl-2-pyridinecarboxylic acid methyl ester (H2)
[0049] Its synthetic reaction is as follows:
[0050]
[0051] In the there-necked flask, install a mechanical stirrer and a thermometer, and dissolve 12.5 grams (0.05mol) of 5-bromo-4-methoxy-2-pyridinecarboxylic acid methyl ester (H2) in 200ml volume ratio (1:2) DMF / In the MeCN solvent, pass through the argon replacement protection, add 44 grams (0.5mol) CuCN, 1 gram of Pd (PPh 3 ) 4 and 20 grams PPh 3 , keep warm at 90°C, detect with TLC until the raw material disappears, stop the reaction, concentrate to dryness, add 50ml 5% NaHCO 3 and 50ml 5% NaCl solution, extracted with 100ml ethyl acetate each time, extracted 3 times altogether, combined organic phase, washed with water to neutrality, dried with anhydrous magnesium sulfate, concentrated by filtration, washed with ethyl acetate and sherwood oil (volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com