Selenophene vinyl compounds and organic conjugated material thereof, and preparation method of organic conjugated material
A technology of selenophene vinyl and compound, applied in the field of selenophene vinyl compound and organic conjugated material and preparation thereof, can solve the problems of complex synthesis steps and high material production cost, achieve fewer synthesis steps, low production cost and wide application Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment provides a kind of selenophene compound, the general structural formula is as shown in formula (I);
[0037]
[0038] The Et is ethyl.
[0039] The present embodiment provides the method for preparing the selenophene compound shown in the above formula (I) as follows:
[0040]
[0041] Utilize olefin metathesis reaction, in organic solvent, first prepare the intermediate product shown in formula (Z) by the compound shown in formula (Y), hereinafter referred to as intermediate product Z, then prepare formula (X) by intermediate product Z The selenophenol vinyl compound shown, wherein Et is ethyl.
[0042] The first step: prepare the intermediate product Z from the compound represented by formula (Y).
[0043] First, under nitrogen protection at -78°C, n-butyllithium (1.8mmol; 0.72ml) was slowly dropped into a solution of selenophene (194mg; 1.5mmol) in anhydrous THF (20mL), then warmed to 0°C and stirred React for 30 minutes; then add Cr(CO) at -...
Embodiment 2
[0046] The present embodiment provides monomer M, and its structural formula is as follows:
[0047]
[0048] The preparation method of monomer M is as follows:
[0049]
[0050] Under the protection of nitrogen at -78°C, n-butyllithium solution (0.38mL, 0.94mmol) was slowly dropped into a solution of compound I (160mg, 0.43mmol) in anhydrous THF (50mL), and the mixture was reacted at -78°C 30 minutes, raised to room temperature and reacted for 1 hour; then cooled to -78°C, Me 3 SnCl (0.94mL, 0.94mmol) was slowly added dropwise to the reaction system, slowly raised to room temperature, and stirred for 8 hours. Add KF aqueous solution to quench the reaction, CH 2 Cl 2 Extract (3×50mL), wash with water (3×50mL), organic phase MgSO 4 Dry and concentrate to obtain a crude product, which is recrystallized from methanol to obtain 211 mg of a red crystalline solid with a yield of 70%.
Embodiment 3
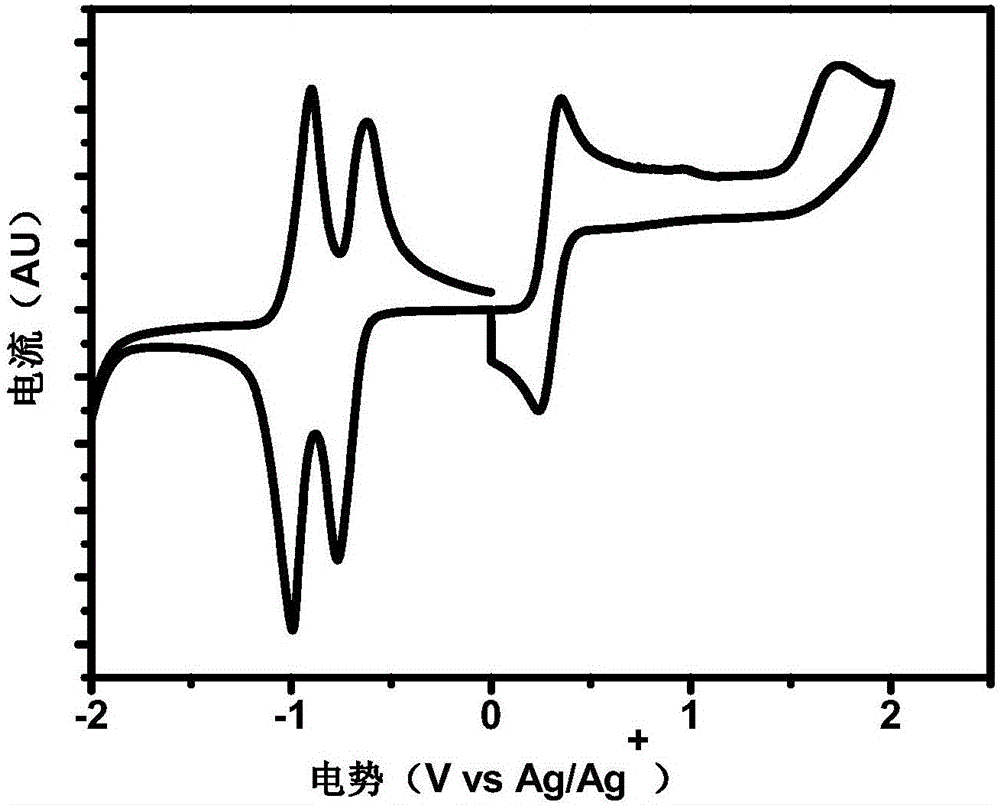
[0052] This embodiment provides a structure of the conjugated polymer material PNDISVSOET as follows:
[0053]
[0054] The preparation method of the conjugated polymer material PNDISVSOET is as follows:
[0055] in N 2 Under gas protection, monomer a (128.1 mg, 0.13 mmol), monomer M (91 mg, 0.13 mmol), Pd 2 (dba) 3 (8mg), P(O-Tol) 3 (11mg), added anhydrous toluene (7mL), heated to 130°C for 48 hours. Add 2-(tributyltin)thiophene, react at 130°C for 6 hours; add 2-iodothiophene, react at 130°C for 6 hours, and cool to room temperature. The reactant solution was poured into methanol (500ml) for coagulation, and the crude product was obtained by filtration. The crude product was sequentially extracted with acetone, n-hexane, THF and chloroform with a Soxhlet extractor. The chloroform extract was concentrated and precipitated in methanol to obtain 106.1 mg of a dark green solid with a yield of 67.9%.
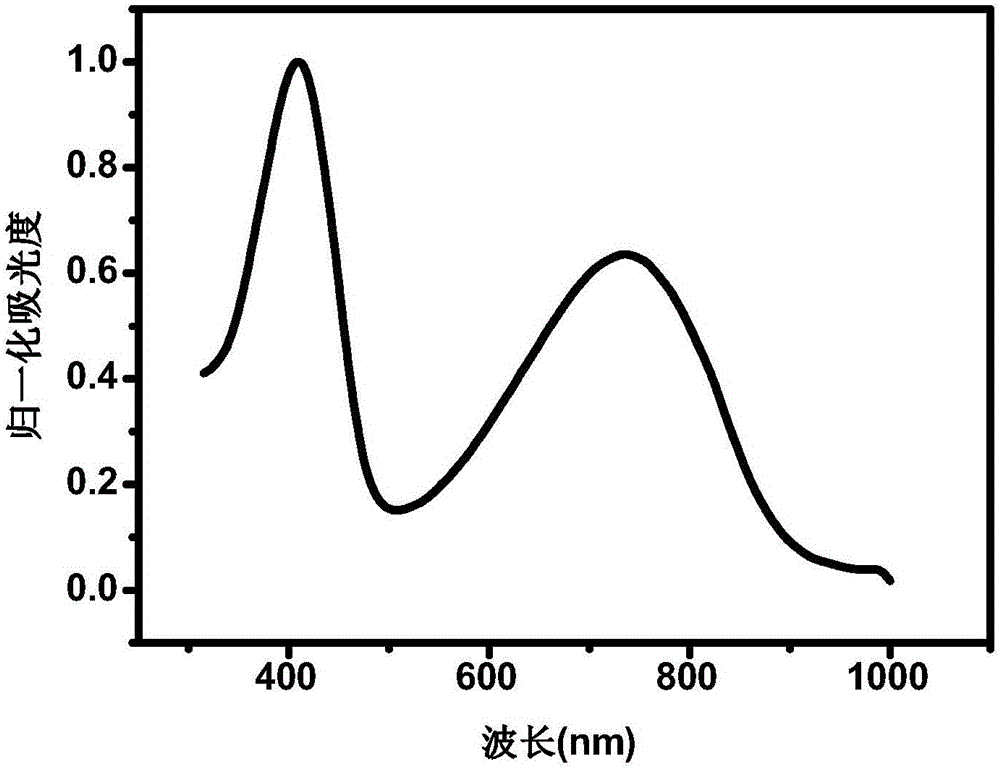
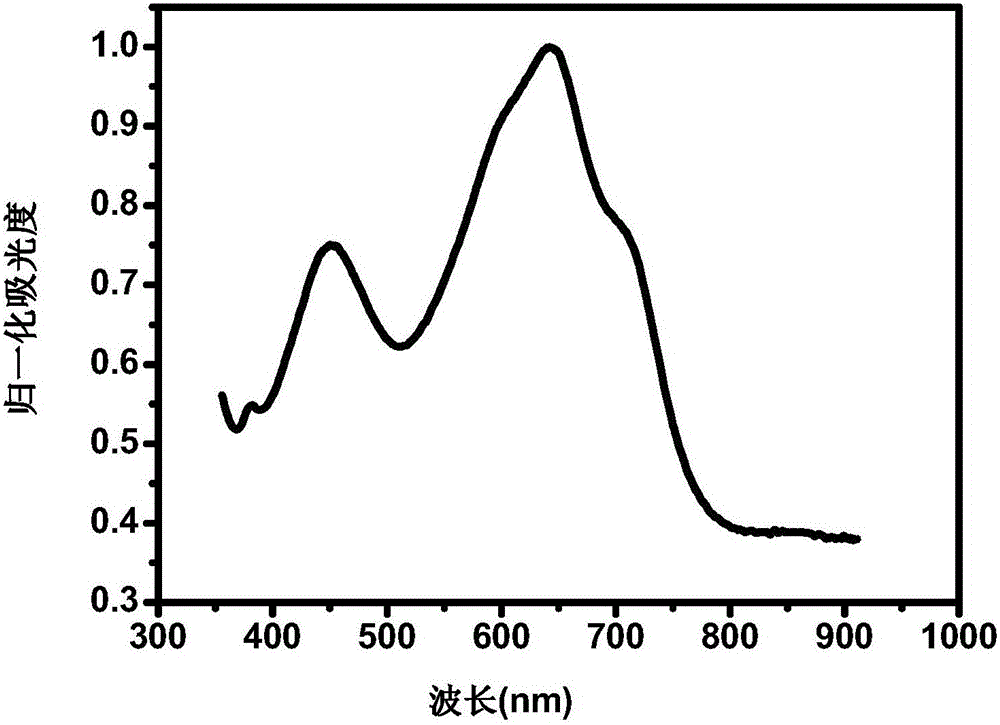
[0056] Gained copolymer PNDISVSOET has wider absorption at 350-1000nm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com